Indexing & Abstracting
Full Text
Review ArticleDOI Number : 10.36811/cjn.2021.110004Article Views : 86Article Downloads : 71
Downregulation of ephrin-B1 is a critical event of podocyte injury
Yoshiyasu Fukusumi, Veniamin Ivanov, Ying Zhang, Hidenori Yasuda and Hiroshi Kawachi*
Department of Cell Biology, Kidney Research Center, Niigata University Graduate School of Medical and Dental Sciences, Japan
*Corresponding Author: Hiroshi Kawachi, MD, PhD, Department of Cell Biology, Kidney Research Center, Niigata University Graduate School of Medical and Dental Sciences, Niigata, 951-8510, Japan, Phone: +81-25-227-2159, Fax: +81-25-227-0770; Email: kawachi@med.niigata-u.ac.jp
Article Information
Aritcle Type: Review Article
Citation: Yoshiyasu Fukusumi, Veniamin Ivanov, Ying Zhang, et al. 2021. Downregulation of ephrin-B1 is a critical event of podocyte injury. Clinic J Nephro. 3: 01-06.
Copyright: This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Copyright © 2021; Hiroshi Kawachi
Publication history:
Received date: 17 June, 2021Accepted date: 29 June, 2021
Published date: 30 June, 2021
Abstract
Proteinuria in several glomerular diseases results from dysfunction of the slit diaphragm, a cell-cell junction of glomerular epithelial cells (podocytes). Ephrin-B1 and its related molecule NHERF 2 are novel essential components of the slit diaphragm. Ephrin-B1 interacts with nephrin via the extra-cellular domain and interacts with NHERF2 via the cytoplasmic site. In the proteinuric state induced by the stimulation to nephrin, nephrin and ephrin-B1 are phosphorylated, and NHERF2 is de- phosphorylated and consequent disruption of the linkage and downregulation of nephrin, ephrin-B1 and NHERF2 are a critical pathogenic event of podocyte injury.
Keywords: Podocyte; Slit Diaphragm; Ephrin-B1; Nephrin; NHERF2
Introduction
The elucidation of the pathogenic mechanism of proteinuria is one of the most important themes in nephrology field. Proteinuria in several glomerular diseases results from dysfunction of the slit diaphragm connecting the neighboring foot processes of glomerular visceral epithelial cell (podocyte) [1-3]. However, the mechanisms of the slit diaphragm injury were not fully clarified yet. In 2007, we reported that ephrin-B1 is expressed at the slit diaphragm and interacts with nephrin, a key molecule of the extra-cellular components of the slit diaphragm [4]. Then, we reported that if nephrin is stimulated, not only nephrin but also ephrin-B1 is evidently downregulated [5]. Recently, we reported that ephrin-B1 interacts with NHERF2, and NHERF2 is also downregulated by the stimulation to nephrin [6]. These reports demonstrated that ephrin-B1 is a crucial molecule for maintaining slit diaphragm function. In this article, first, we overview the molecular composition of the slit diaphragm and discuss the role of ephrin-B1 and its related molecule NHERF2 of the slit diaphragm in physiological and pathological states.
Overview: molecular structure of slit diaphragm
The first molecule identified as a slit diaphragm component is zonula occludens 1 (ZO-1). ZO-1 was originally identified as a component of tight junction [7] and was observed to be concentrated along the cytoplasmic surface of the slit diaphragm [8]. A key molecule maintaining the barrier function of the slit diaphragm is nephrin. Nephrin was identified as a product of a gene mutated in familial steroidresistant nephrotic syndrome (NPHS1) [9]. Another key molecule of the slit diaphragm is podocin. Podocin was identified as a gene product of NPHS2, the causative gene of autosomal recessive steroid-resistant nephrotic syndrome [10]. Podocin interacts with nephrin and CD2AP [11]. CD2AP is also a functional molecule of the slit diaphragm [12]. CD2AP is an adaptor molecule that was identified to bind to CD2, a membrane protein on T cells [13]. CD2AP was shown to interact with nephrin and could anchor nephrin to the cytoskeleton [12]. It is reported that some patients with focal segmental glomerular sclerosis had a mutation of CD2AP [14]. NEPH1 was identified as a nephrin-related protein with a technique of the gene trapping screen [15]. NEPH1 interacts with ZO-1 and nephrin [16].
Ephrin-B1, a novel critical component of the slit diaphragm
To explore a novel critical molecule that participates in the maintenance of the function of the slit diaphragm, we intended to purify the molecules whose expressions were downregulated in the proteinuric state resulted from slit diaphragm dysfunction. It is conceivable that the molecules downregulated in the proteinuric state might be a functional molecule of the slit diaphragm. Ephrin-B1 was identified as a molecule of which mRNA expression is decreased in the nephrotic state induced by the stimulation to nephrin [4]. Ephrin and its related protein Eph function as receptor-ligand pairs [17- 19]. The Eph-ephrin family is reported to have many biological functions, such as formations of tissue-border, vascular development, and cell migration [20- 22]. It is also reported that the Eph-ephrin-B family regulates the paracellular permeability of epithelial cells [20]. Dual labeling immunohistochemical analyses with a normal rat glomerular section showed that ephrin-B1 is colocalized with nephrin (Figure 1), which indicates that ephrin-B1 is a component of the slit diaphragm. Podocyte-specific ephrin-B1 conditional knockout (CKO) mice display clear alterations of the podocyte morphology, disarrangement of the critical molecules of the slit diaphragm, and the mice showed proteinuria. The in-vitro analyses with the HEK293 cell expression system revealed ephrin-B1 interacts with nephrin via the extracellular domain [5]. It was concluded that ephrin-B1 is one of the critical components of the slit diaphragm and is essential for the maintenance of the integrity of the slit diaphragm.
NHERF2, an ephrin-B1-associated molecule
Then, we explored ephrin-B1-related molecules, then we found mRNA expression of NHERF2 was decreased in podocyte-specific ephrin-B1 CKO mice. The observation suggested that NHERF2 is a ephrin-B1-related molecule [6]. NHERF2, an isoform of NHERF proteins, was initially identified by two-hybrid screening using the cytoplasmic tail of Na+/H+-exchanger 3 (NHE3) [23]. Takeda et al. reported that NHERF2 interacts with podocalyxin, which is expressed at the apical surface of podocytes [24]. Dual-labeling immunohistochemical analyses with normal rat kidney section showed that some portions of NHERF2 are colocalized with ephrin-B1 (Figure 1). The interaction assay with HEK293 cells showed that NHERF2 interacts with ephrin-B1. These observations implied that NHERF2 is a component of the slit diaphragm complex.

Figure 1: Dual-labeling immunofluorescence of ephrin-B1 with nephrin and NHERF2. Ephrin-B1 is almost completely colocalized with nephrin (detected as yellow) (upper panel). Ephrin-B1 is colocalized with NHERF2 (seen as yellow), and some portions of NHERF2 are apart from ephrin-B1 (detected as green) (lower panel).
Ephrin-B1 and NHERF2 are downregulated in the proteinuric state caused by the stimulation to nephrin
Immunostaining of ephrin-B1 and NHERF2, as well as nephrin, are evidently downregulated in rat nephrotic model induced by the injection with the antibody against nephrin [4-6] (Figure 2). The invitro analyses with the HEK293 cell expression system showed that if nephrin was stimulated by the antibody against the extracellular site of nephrin, nephrin and ephrin-B1 were tyrosine-phosphorylated, and serine/threonine of NHERF2 was de-phosphorylated, and the linkage of nephrin-ephrin-B1- NHERF2 was disrupted [5-6]. Thus, the observations implied that altered phosphorylated state of nephrin, ephrin-B1, and NHERF2 and downregulation of these molecules are involved in developing the slit diaphragm injury.
Conclusions
Ephrin-B1 and NHERF2 are essential components of the slit diaphragm of glomerular podocytes. The interactions of ephrin-B1 with nephrin and NHERF2 are essential for maintaining the proper molecular structure and the function of the slit diaphragm. Tyrosine phosphorylation of ephrin-B1 and consequent de-phosphorylation of serine/threonine of NHERF2 induced by the stimulation to nephrin leads to the disruption of the linkage of nephrin-ephrin-B1-NHERF2, and downregulation of these molecules is a critical pathogenic event of podocyte injury. A schematic diagram of the proposed pathogenic mechanism is shown in Figure 3.
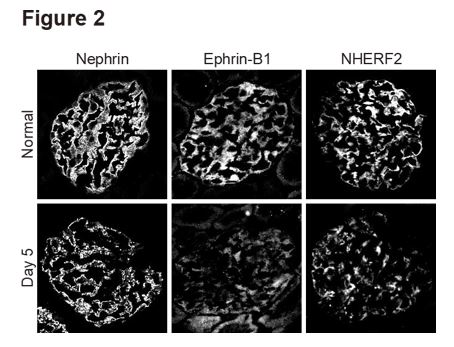
Figure 2: Immunostaining of nephrin, ephrin-B1 and NHERF2 in proteinuric state of anti-nephrin antibody-induced nephropathy. Immunostaining intensity of nephrin, ephrin-B1, and NHERF2 is evidently decreased, and their staining pattern changed to be discontinuous on day 5 when proteinuria peaked.
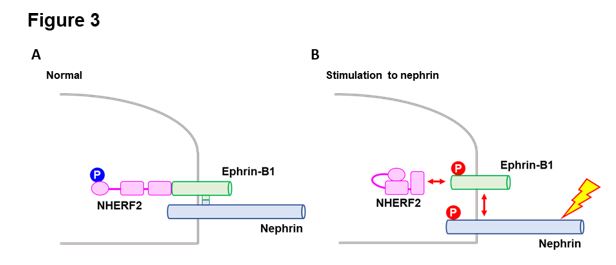
Figure 3: Schematic diagram of the proposed pathogenic mechanism.
A: Ephrin-B1 interacts with nephrin via the extracellular domain and interacts with NHERF2 via the cytoplasmic site. B: If nephrin is stimulated, nephrin and ephrin-B1 are phosphorylated, and NHERF2 is de-phosphorylated, and consequently, the linkage of nephrin-ephrin-B1-NHERF2 is disrupted.
References
1. Pavenstadt H, Kriz W, Kretzler M. 2003. Cell biology of the glomerular podocyte. Physiol Rev. 83: 253-307. Ref.: https://pubmed.ncbi.nlm.nih.gov/12506131/ DOI: https://doi.org/10.1152/physrev.00020.2002
2. Holthofer H. 2007. Molecular architecture of the glomerular slit diaphragm: lessons learnt for a better understanding of disease pathogenesis. Nephrol Dial Transplant. 22: 2124-2128. Ref.: https://pubmed.ncbi.nlm.nih.gov/17550922/ DOI: https://doi.org/10.1093/ndt/gfm344
3. Kawachi H, Shimizu F. 2000. Molecular composition and function of the slit diaphragm: nephrin, the molecule responsible for proteinuria. Clin Exp Nephrol. 4: 161-172.
4. Hashimoto T, Karasawa T, Saito A, et al. 2007. Ephrin-B1 localizes at the slit diaphragm of the glomerular podocyte. Kidney Int. 72: 954-964. Ref.: https://pubmed.ncbi.nlm.nih.gov/17667985/ DOI: https://doi.org/10.1038/sj.ki.5002454
5. Fukusumi Y, Zhang Y, Yamagishi R, et al. 2018. Nephrin-Binding Ephrin-B1 at the Slit Diaphragm Controls Podocyte Function through the JNK Pathway. J Am Soc Nephrol. 29: 1462-1474. Ref.: https://pubmed.ncbi.nlm.nih.gov/29602834/ DOI: https://doi.org/10.1681/asn.2017090993
6. Fukusumi Y, Yasuda H, Zhang Y, et al. 2021. Nephrin-Ephrin-B1-Na(+)/H(+) Exchanger Regulatory Factor 2-Ezrin- Actin Axis Is Critical in Podocyte Injury. Am J Pathol. 191: 1209-1226. Ref.: https://pubmed.ncbi.nlm.nih.gov/33887216/ DOI: https://doi.org/10.1016/j.ajpath.2021.04.004
7. Anderson JM, Stevenson BR, Jesaitis LA, et al. 1988. Characterization of ZO-1, a protein component of the tight junction from mouse liver and Madin-Darby canine kidney cells. J Cell Biol. 106: 1141-1149. Ref.: https://pubmed.ncbi.nlm.nih.gov/2452168/ DOI: https://doi.org/10.1083/jcb.106.4.1141
8. Schnabel E, Anderson JM, Farquhar MG. 1990. The tight junction protein ZO-1 is concentrated along slit diaphragms of the glomerular epithelium. J Cell Biol. 111: 1255-1263. Ref.: https://pubmed.ncbi.nlm.nih.gov/2202736/ DOI: https://doi.org/10.1083/jcb.111.3.1255
9. Kestila M, Lenkkeri U, Mannikko M, et al. 1998. Positionally cloned gene for a novel glomerular protein--nephrin--is mutated in congenital nephrotic syndrome. Mol Cell. 1: 575-582. Ref.: https://pubmed.ncbi.nlm.nih.gov/9660941/ DOI: https://doi.org/10.1016/s1097-2765(00)80057-x
10. Boute N, Gribouval O, Roselli S, et al. 2000. NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet. 24: 349-354. Ref.: https://pubmed.ncbi.nlm.nih.gov/10742096/ DOI: https://doi.org/10.1038/74166
11. Schwarz K, Simons M, Reiser J, et al. 2001. Podocin, a raft-associated component of the glomerular slit diaphragm, interacts with CD2AP and nephrin. J Clin Invest. 108: 1621-1629. Ref.: https://pubmed.ncbi.nlm.nih.gov/11733557/ DOI: https://doi.org/10.1172/jci12849
12. Shih NY, Li J, Karpitskii V, et al. 1999. Congenital nephrotic syndrome in mice lacking CD2-associated protein. Science. 286: 312-315. Ref.: https://pubmed.ncbi.nlm.nih.gov/10514378/ DOI: https://doi.org/10.1126/science.286.5438.312
13. Dustin ML, Olszowy MW, Holdorf AD, et al. 1998. A novel adaptor protein orchestrates receptor patterning and cytoskeletal polarity in T-cell contacts. Cell. 94: 667-677. Ref.: https://pubmed.ncbi.nlm.nih.gov/9741631/ DOI: https://doi.org/10.1016/s0092-8674(00)81608-6
14. Kim JM, Wu H, Green G, et al. 2003. CD2- associated protein haploinsufficiency is linked to glomerular disease susceptibility. Science. 300: 1298-1300. Ref.: https://pubmed.ncbi.nlm.nih.gov/12764198/ DOI: https://doi.org/10.1126/science.1081068
15. Donoviel DB, Freed DD, Vogel H, et al. 2001. Proteinuria and perinatal lethality in mice lacking NEPH1, a novel protein with homology to NEPHRIN. Mol Cell Biol. 21: 4829-4836. Ref.: https://pubmed.ncbi.nlm.nih.gov/11416156/ DOI: https://doi.org/10.1128/mcb.21.14.4829-4836.2001
16. Liu G, Kaw B, Kurfis J, et al. 2003. Neph1 and nephrin interaction in the slit diaphragm is an important determinant of glomerular permeability. J Clin Invest. 112: 209-221. Ref.: https://pubmed.ncbi.nlm.nih.gov/12865409/ DOI: https://doi.org/10.1172/jci18242
17. 1997. Unified nomenclature for Eph family receptors and their ligands, the ephrins. Eph Nomenclature Committee. Cell. 90: 403- 404. Ref.: https://pubmed.ncbi.nlm.nih.gov/9267020/ DOI: https://doi.org/10.1016/s0092-8674(00)80500-0
18. Kullander K, Klein R. 2002. Mechanisms and functions of Eph and ephrin signalling. Nat Rev Mol Cell Biol. 3: 475-486. Ref.: https://pubmed.ncbi.nlm.nih.gov/12094214/ DOI: https://doi.org/10.1038/nrm856
19. Pasquale EB. 2005. Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Mol Cell Biol. 6: 462-475. Ref.: https://pubmed.ncbi.nlm.nih.gov/15928710/ DOI: https://doi.org/10.1038/nrm1662
20. Martinez A, Soriano E. 2005. Functions of ephrin/Eph interactions in the development of the nervous system: emphasis on the hippocampal system. Brain Res Brain Res Rev. 49: 211-226. Ref.: https://pubmed.ncbi.nlm.nih.gov/16111551/ DOI: https://doi.org/10.1016/j.brainresrev.2005.02.001
21. Poliakov A, Cotrina M, Wilkinson DG. 2004. Diverse roles of eph receptors and ephrins in the regulation of cell migration and tissue assembly. Dev Cell. 7: 465-480. Ref.: https://pubmed.ncbi.nlm.nih.gov/15469835/ DOI: https://doi.org/10.1016/j.devcel.2004.09.006
22. Cheng N, Brantley DM, Chen J. 2002. The ephrins and Eph receptors in angiogenesis. Cytokine Growth Factor Rev. 13: 75-85. Ref.: https://pubmed.ncbi.nlm.nih.gov/11750881/ DOI: https://doi.org/10.1016/s1359-6101(01)00031-4
23. Yun CH, Oh S, Zizak M, et al. 1997. cAMP-mediated inhibition of the epithelial brush border Na+/H+ exchanger, NHE3, requires an associated regulatory protein. Proc Natl Acad Sci U S A. 94: 3010-3015. Ref.: https://pubmed.ncbi.nlm.nih.gov/9096337/ DOI: https://doi.org/10.1073/pnas.94.7.3010
24. Takeda T, McQuistan T, Orlando RA, et al. 2001. Loss of glomerular foot processes is associated with uncoupling of podocalyxin from the actin cytoskeleton. J Clin Invest. 108: 289-301. Ref.: https://pubmed.ncbi.nlm.nih.gov/11457882/ DOI: https://doi.org/10.1172/jci12539




















