Indexing & Abstracting
Full Text
Mini ReviewDOI Number : 10.36811/ijapr.2019.110001Article Views : 2466Article Downloads : 33
Botulinum Toxin Treatment for Painful Diabetic Neuropathy A Review
Bahman Jabbari1* and Yasaman Safarpour2
1Yale University School of Medicine, USA
2University of California, Irvine, USA
*Corresponding author: Bahman Jabbari M.D, Yale University School of Medicine, USA, Tel: 914-482-8542; Email: Bahman.jabbari@yale.edu
Article Information
Aritcle Type: Mini Review
Citation: Bahman Jabbari, Yasaman Safarpour. 2019. Botulinum Toxin Treatment for Painful Diabetic Neuropathy A Review. Int J Anesthesi Pain Res. 1: 01-04.
Copyright:This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Copyright © 2019; Bahman Jabbari
Publication history:
Received date: 16 March, 2019Accepted date: 23 March, 2019
Published date: 25 March, 2019
Introduction
Diabetic neuropathy (DN) is one of the most common peripheral nervous system disorders. It affects 16% of individuals with type I (young onset) diabetes and 25-26% of individuals with type II (late onset) diabetes [1]. Pain and numbness of the feet and, in advanced cases, weakness in the feet or hands are the usual symptoms. These symptoms are typically more prominent in the lower limbs. The skin in the affected areas is sensitive to touch (hyperesthesia); sometimes touch evokes pain (allodynia). The pain of diabetic neuropathy may develop spontaneously or may be provoked by touch or motion. Pain often interferes with patient’s rest and sleep and has typical characteristics of a neuropathic pain i.e having a sharp and burning quality. Dorsum of the foot and toes are most commonly affected in diabetic neuropathy. On examination, the patients demonstrate decreased sensations (heat, cold, touch, position) in the affected limb. Diabetic neuropathy (DN) is usually bilateral and presents in form of a polyneuropathy.
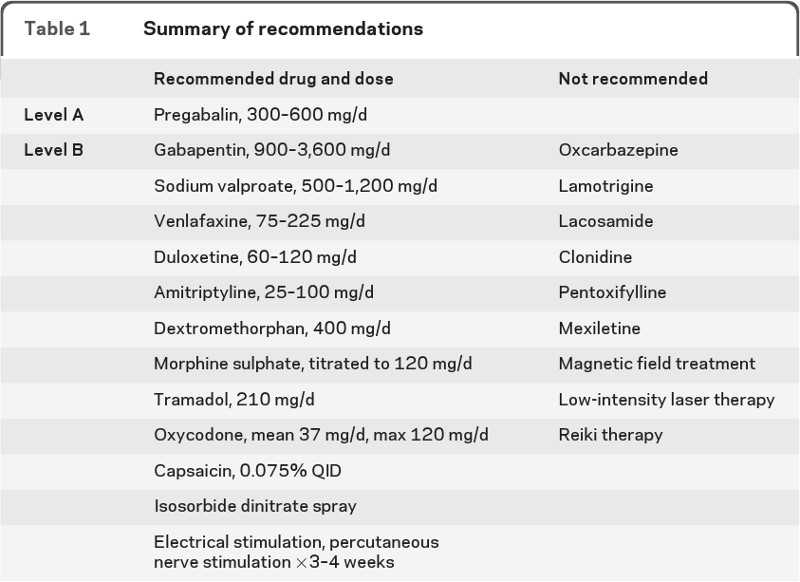
Treatment of DN consists of avoiding sugar, lowering serum glucose levels with medications and treating pain. Both pharmacological and non-pharmacological measures have been employed to manage the pain in DN. Table 1 shows recommended and not-recommended approaches according to the guidelines published by the American academies of Neurology and Physical Medicine and Rehabilitation [2]. The levels of efficacy in this table (A: definitely effective, B: probably effective) are based on published guidelines (A level: requiring two class I and B level: requiring one class I or two class II studies) [3].
From Bril et al. [3], - PMR 2011
In more recent years, other agents have been found helpful in relieving the pain of DN in both humans and animal models. In human, the efficacy cannabinoids inhalation [4], to relive the pain of diabetic neuropathy has been shown in a small randomized, double blind, placebo-controlled study. In rats, several studies suggested that epigallocatechin-3-gallate (EGCG), the active component of green tea, can relieve neuropathic pain including the pain of DN [5].
Botulinum Toxin Treatment of Pain in Diabetic Neuropathy (DN)
In animal models of local pain, injection of the botulinum toxin A or B into the skin of the painful area can relieve pain and block local accumulation of pain neurotransmitters such as glutamate and calcitonin gene related peptides [6]. Apart from the peripheral mechanism, presence of botulinum toxins A and B target proteins in spinal cord sensory neurons after peripheral injection, strongly suggests an independent central mechanism [7]. OnabotulinumtoxinA (Botox) was approved for chronic migraine in Europe and the US in 2010. In recent years, a large number of clinical trials have strongly suggested the efficacy of botulinum toxins in a variety of pain disorders [6].
Method of the Review
The published literature on the use of botulinum toxins in postherpetic neuralgia was reviewed, using Medline and Ovis SP search engines. The search included all English manuscripts published between January 1,1989 (the year botulinum toxin was introduced into the market) to February 1st ,2019. The search words included botulinum toxin, botulinum neurotoxin, diabetes, diabetic neuropathy and neuropathic pain.
Results
This search identified a total of forty five manuscripts, of which 5 manuscripts were directly relevant to the search subject (Table 2). Four manuscripts represented, randomized, double-blind, placebo-controlled clinical trials. Three of these four studies were of parallel design, while one had a cross-over design. All studies used type A toxin- three used Botox, one Xeomin and one Dysport. Of four studies with neuropathic pain (foot), the mode of injection was intradermal in three; the toxin was employed in small doses using a grid-like pattern over the dorsum of the foot. In one patient, the toxin (Botox) was injected directly into the lumbar plexus. One double - blind study evaluated the efficacy of intramuscular toxin injection in relieving calf and foot cramps associated with diabetic neuropathy (table 2). The toxin dose used was 100 units in four and 50 units in one study. The response of neuropathic pain was evaluated by visual analogue scale (0-10) and that of muscle cramps by changes in frequency and intensity of cramps. All 4 studies of neuropathic pain in DN demonstrated significant pain relief after botulinum toxin treatment. In the study of Restivo et al. [7,8], intramuscular injection of the toxin reduced the frequency and intensity of cramps substantially. None of the studies demonstrated any serious adverse effects.
| Table 2: Published manuscripts on the subject of BoNT treatment of painful diabetic neuropathy. | ||||
|---|---|---|---|---|
| Authors and date | Type of study | Number of patients | Toxin and dose in units(U), mode of injection | Results |
| Restivo DA et al. 2018 [9] | Double blind, Placebo controlled, parallel design | 50 | Xeomin 30 or 100 U IM | Significant reduction of intensity and frequency of calf and foot cramp in the toxin group |
| Moon et. al.2016 | Single case | 1 | Botox, 100 units- Lumbar plexus | Significant pain reduction for 4-5 months. |
| Ghasemi et al. 2014 [10] | Double blind, placebo controlled, parallel design | 40 | Dysport, 100 U, ID | 30% of the patients in toxin group became pain free (P<0.001). Except cold, all sensations improved |
| Chen et. 2013 [11] | Double blind, placebo controlled, parallel design | 18 | Botox 50 units ID | Significant reduction of tactile and mechanical sensation in the toxin group |
| Yuan et al. 2009 [12] | Double blind placebo controlled, cross-over design | 18 | Botox, 100 units ID | At 4 and 12 weeks, Toxin group demonstrated significant decrease in VAS score. |
| VAS: Visual analogue Scale - SC: subcutaneous- ID: intradermal - IM: intramuscular. | ||||
Conclusion
The data from high quality studies of Botulinum toxin A in DN is encouraging and suggest the efficacy of all type A toxins in relieving both neuropathic pain and painful cramps in DN. Lack of significant adverse effects, prolonged effect (3-4 months) after one set of injections versus daily use of oral analgesic agents, makes botulinum toxin treatment desirable to patients. Larger randomized clinical trials are necessary to support this conclusion and demonstrate the long-term efficacy and safety of botulinum toxin treatment in painful diabetic neuropathy.
References
- Barrett AM, Lucero MA, Le T, et al. 2007. Epidemiology, public health burden, and treatment of diabetic neuropathic pain: a review. Pain Med. 8: 50-62. [Ref.]
- Bril V, England J, Franklin GM, et al. 2011. Evidence-based Guideline: Treatment of painful diabetic neuropathy report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. PMR. 3: 345-352. [Ref.]
- American Academy of Neurology. Clinical Practice Guidelines Process Manual, 2004 ed, St Paul, MN: available at: https;//www.aan.com/Guidelines/Home/UnderDevelopment. [Ref.]
- Wallace MS, Marcotte TD, Umlauf A, et al. 2015. Efficacy of Inhaled Cannabis on Painful Diabetic Neuropathy. J Pain. 16: 616-627. [Ref.]
- Bimonte S, Cascella M, Schiavone V, et al. 2017. The role of Epigallocatechin-3-gallate in treatment of neuropathic pain: an update on preclinical in vivo studies and future perspectives. Drug Des Devel There. 11: 2737-2742. [Ref.]
- Marino MJ, Terashima T, Steinauer JJ, et al. 2014. Botulinum toxin B in the sensory afferent: Transmitter release, spinal activation, and pain behavior. Pain. 155: 674-684. [Ref.]
- Matak I, Riederer P, Lackovi? Z. 2012. Botulinum toxin's axonal transport from periphery to the spinal cord. Neurochem Int. 61:236-239. [Ref.]
- Jabbari B. Botulinum toxin in Pain Disorders. Springer Publisher. New York 2015. [Ref.]
- Restivo DA, Casabona A, Frittitta L, et al. 2018. Efficacy of Botulinum toxin A for treating cramps in diabetic neuropathy Ann Neurol. 84: 682-690. [Ref.]
- Ghasemi M, Ansari M, Basiri K, et al. 2014. The effects of intradermal botulinum toxin type a injections on pain symptoms of patients with diabetic neuropathy. J Res Med Sci. 19: 106-111. [Ref.]
- Chen WT, Yuan RY, Chiang SC, et al. 2013. OnabotulinumtoxinA improves tactile and mechanical pain perception in painful diabetic neuropathy. Clin J Pain. 29: 305-310. [Ref.]
- Yuan RY, Sheu JJ, Yu JM, et al. 2009. Botulinum toxin for diabetic pain: a randomized, double- blind, cros-over trial. Neurology. 72: 1473-1478.[Ref.]




















