Indexing & Abstracting
Full Text
Research ArticleDOI Number : 10.36811/ijbm.2019.110014Article Views : 136Article Downloads : 72
Effect of nanoparticles on Escherichia coli growth dynamics
Laith B Alhusseini
Department of Environment, College of Science, University of Kufa, Najaf, Iraq
*Corresponding author: Laith B Alhusseini, Department of Environment, College of Science, University of Kufa, Najaf, Iraq, Tel: 00962798969516; Email: alhusainilaith@gmail.com
Article Information
Aritcle Type: Research Article
Citation: Laith B Alhusseini. 2019. Effect of nanoparticles on Escherichia coli growth dynamics. Int J Biol Med. 1: 107-113.
Copyright: This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Copyright © 2019; Laith B Alhusseini
Publication history:
Received date: 11 May, 2019Accepted date: 18 July, 2019
Published date: 19 July, 2019
Abstract
Background: Nanoparticles (iron oxide and titanium dioxide nanoparticles) are another kind of critical materials that are produced for use in various research and different purposes. The bacteriology field being so critical seek to the intrinsic understanding on the effect of nanoparticles on bacterial growth and functions. Our investigation was planned to detect the impact of iron oxide (Fe3O4), titanium dioxide (TiO2) nanoparticles on growth of Escherichia coli (Iraqi isolate).
Methods: Fifty urine samples of patients, who are suffering Urinary Tract Infections (UTIs) in Iraqi hospitals, were collected. Our study was included three parts: the 1st part was isolated and diagnosed the bacteria that cause the urinary tract infection, the 2nd part was sensitivity to antibiotics, and the 3rd has used the nanomaterials and study their impacts on the growth of E. col isolates.
Result: The results showed that 30 E. coli isolates depending on the properties of biochemical and molecular detect. Five common types of antibiotics were examined for the treatment of infections of the urinary tract. Most E. coli were resistant to antibiotics, the ratios of ampicillin, amikacin and augmentin found to be 90%, 82% and 80% respectively. It concluded that bacteria were sensitive to imipenem and meropenem of about 50 %. So, the effect of iron oxide and titanium dioxide nanoparticles were studied for the growth of bacteria using the agar. The effectiveness against bacteria (diameters of the inhibition zone rate) found to be 18 mm for the 1st substance and 21 mm for 2nd substance.
Conclusion: Our current study indicates that there is an effect of nanoparticles at the cellular level that can be used for beneficial biological application such as antibacterial.
Keywords: Escherichia coli; Inhibition zone; Antibiotics; Nanoparticles
Introduction
Escherichia coli (E.coli) is the important type of the Enterobacteriaceae, which includes close races between them E. coli and related bacteria are gut flora as endemic naturally in the gut of humans and other animals[1] . Facultative anaerobic bacteria, which is gram-negative rod [2], grow anaerobically and is produced acids and gas [3]. These bacteria were discovered by Theodor Escherichia in (1885) [4]. E. coli bacteria is the main reason for urinary tract infections, especially in pregnant women, with the percentage of the injury of about 90% [5]. Moreover, it is one of the most important causes of diarrhea in children, travelers, and chronic diarrhea [6]. The bacteria caused by the infections of the urinary tract has many virulences of its factor’s adhesives and hemolysin enzyme and urease enzyme, capsule, which enable it to the events of infection. Antibiotics are commonly affected the lowest average of infections of the urinary tract, but because of the indiscriminate used to antibiotics and the refrain from the treatment without consulting a doctor. Bacterial strains resistant to antibiotics have emerged, the qualities of bacterial resistance were spread increasingly with the increased of antibiotics and then the treating of diseases will be difficult. So must test the antibiotic appropriate for their treatment and not be treated randomly, but rather it depends on a drug sensitivity with the bacteria for determining the appropriate antibiotic for killing it [7,8]. Moreover, the present era belongs to nanotechnology. Nanotechnology has become a major interdisciplinary theme as a result of the rapid growth of science. The application and development of nanotechnology have the sc0pe to significantly improve the quality of life. For example, nanoparticles gold is mostly nanoparticles used in biomedical sciences [9]. Because of their biological compatibility and magnetic properties [10,11]. Furthermore, several factors such as shape, size, synthesis and composition etc. have various conclusions even for related nanoparticles [12,13]. Current study included the effect of inhibition of nanoparticles on the growth of bacteria (inhibition zone) and compared with some antibiotics.
Materials and Methods
Collection of isolates
Current study, more than fifty-five isolates diagnosed as E. coli were collected from clinical samples. Urine samples were collected from patients Suffering from UTI urinary tract infection in some Baghdad hospitals [14].
Diagnosis of E coli
For diagnosis isolates, the API 20 E system has been used, a number of researchers have used of this diagnostic system [15].
Antimicrobial susceptibility test
Antibiotic sensitivity method was done on Mueller-Hinton agar (MHA) by Kirby–Bauer disc diffusion method [16]. using following antibiotic discs (Bioanalyze, turkey ) ampicillin (AMP 10 mcg), amikacin (AK 30 mcg), amoxicillin-clavulanic acid (AMC 30 mcg), meropenem (MEM 10 mcg), imipenem (IPM 10 mcg), Since the resistance and sensitivity were determined based on the standard diameters by CLSI guidelines 2013.
Synthesis of nanoparticles
Iron oxide nanotubes were prepared using the anodizing method, as shown in Fig. 2, using electrochemical deposition (cell dimensions of about 50 × 70mm) [17,18]. The microwave oven was used to prepare nanotubes of titanium dioxide (TiO2), as shown in Fig. 3. Twenty-two grams (TiO2) were added to 50 ml of 18 M of potassium hydroxide. The solution was put in beaker pyrex glass (steel and high energy). The solution is heated using a microwave oven with high-energy of 1200 watts at 20 m. Then it was washed with distilled water then dried in the oven at 100oC for 5 h [19]. Well-diffusion was used to detect the effectiveness of nanomaterials against isolates in the study. Then 0.1 ml of cultivated bacteria was distributed using sterile glass. The hole with a diameter of 5 mm has been done at the center surface. Each hole was filled with nanomaterials of about 50 μL and is incubated at 37oC, then inhibition zones around the drilling were measured [20].
Results and Discussion
Seventy-five bacterial isolates from UTI collected were taken from patients in Baghdad hospitals. As it clears all the 50 isolates (66.66%) gave a positive result in this API 20E test which may give a precise idea that the isolates were E. coli. Figure (1) showing the results of the tests for E. coli using the API 20 E System.

Figure 1: Results of the API 20 E System.
The sensitivity test of E. coli isolates of antibiotics was performed by Kirby–Bauer disc diffusion method on Mueller-Hinton agar [16]. five different antibiotics Ampicillin, Amikacin, Augmentin Meropenem and Impenem were used to measure the diameter of inhibition in millimeters around the antibiotic disc and compared with the standard (CLSI, 2012). The names, symbol concentrations, and areas of growth inhibition of these antibiotics are shown in Table 3.
|
Table 3: The names, symbols of the concentrations (µg), and standard diameters of inhibition zones to antibiotics (mm) [16]. |
|||||
|
Antibiotics |
Symbol |
Concentration |
Diameters of inhibition zones |
||
|
Sensitive |
Medium |
Resistance |
|||
|
Ampicillin |
AM |
10 |
<17 |
14-16 |
>13 |
|
Amikacin |
Ak |
10 |
<26 |
20-25 |
>19 |
|
Augmentin |
AMC |
30 |
<18 |
14-17 |
>13 |
|
Imipenem |
IPM |
10 |
<23 |
20-22 |
>19 |
|
Meropenem |
MEM |
30 |
<23 |
20-22 |
>19 |
Among 50 samples tested, most of E. coli isolates were MDR. The isolates showed high level of resistance to ampicillin (90%), amoxicillin-clavulanic acid (80%), Amikacin (82%) and were sensitive ratio of both meropenem and impenem was 50% on sequentially (50%). In Figure 3 show antibiotic resist ratio.
Figure: Resistance to antibiotics
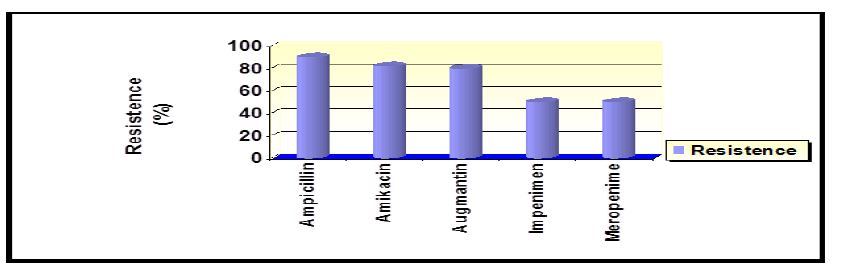
The results were close to (Yaseen, 2014) found that the percentage of bacterial resistance Ampicillin was 100% did not agree the study Amikacin, where the researcher found that the resistance rate is 12.8%. In a study by Rayes et al. (2010) found that the resistance to Impenem was 0%, while the current study found that the resistance ratio is 50%. Our results differed with Al-Attar (2014). It was found that resistance to bacteria Meropenem was 3.3% while our results 50%. The reason for the resistance may be due to the production of bacterial enzyme beta-lactamase encrypted chromosomally [24]. One of the main reasons for the emergence of multi-resistant is the indiscriminate use of antibiotics without relying on its sensitivity, which increases the chances of scalability and adaptation of bacteria resistant to antibiotics used in therapy [25]. It is very common in hospitals is isolated strains resistant to multiple antibiotics, particularly beta-lactam antibiotics within the intestinal family members [26]. Sasaki and Hooper found that 85.1% of the isolates have the ability to produce beta-lactam enzyme. All isolates of E. coli are sensitive to antibiotics of about 89% [27,28].
NPs in have broad-spectrum antibacterial properties against both Gram-positive and Gram-negative bacteria. For example, ZnO NPs nanoparticles reduced the growth of A.baumanii, E.cloacae, P. aeruginosa, E.coli, Klebsiella, Bacillus and K. pneumonia [29,30]. The results showed that the nanomaterials used against these isolates have the inhibition zone (figure 4) rate of 18 mm in diameter using iron oxide nanoparticles. Our findings suggest that iron oxide has anti-bacterial effect and this is consistent with mentioned He S et, al (2011). Increased Fe ion assisted Fe binding with proteins and DNA strands, cussing the mutation frequency of E. coli [31]. While is found to be 21 mm using titanium dioxide nanoparticles. The results suggested that titanium caused the antibacterial effect, our results correlate with the results of Lin X et, al (2014). He found that TiO2 NPs and smaller particle size had higher affinity to the cell surfaces and induced heavier oxidative damage and toxicity to the bacterial cells, and the toxicity decreased with increasing pH (5.0–10.0) and ionic strength (50–200 mg L−1 NaCl) [32].
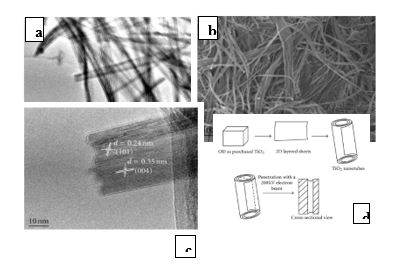
Figure 2: (a) SEM (b) TEM (c) HRTEM micrographs (d) formation mechanism of TiO2 nanotubes.

Figure 3: TEM images of Fe2O3 nanoparticles.
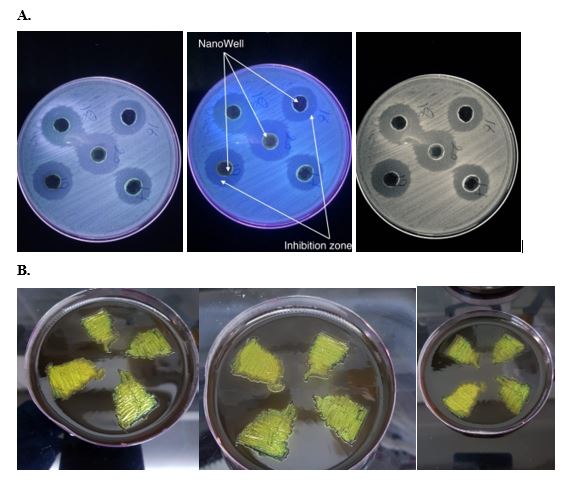
Figure 4: (A) Inhibition zone around nanoparticles. B) E coli on eosin methylene blue agar.
Conclusions
The E. coli has contributed to UTI and other diseases. Multiple antibiotic resistance founded in E. coli. This is a significant obstacle to treatment. In addition, some E. coli isolates the dormant phase during treatment and then return after the disappearance of the concentration of the antibiotic. Therefore, an effective treatment must be developed to eliminate it. The results showed that nanoparticles in this study had clear effects on bacteria. The types of nanomaterials, method of preparation, characteristics, and its focus has an impact on the deadly bacteria. The effect involves destruction of the cell wall, cell membrane, genetic material, cellular protein, etc. Finally, effective alternatives should be found for the disposal of pathogenic bacteria. One of these alternatives may be nanomaterials. And determine the effect of nanoparticles on each type of bacteria. The genome study is more important for genetic location determination, and is the director of multiple resistance to antibiotics.
Acknowledgment
The author acknowledges the financial support of the College of Science of the University of Kufa. The author is grateful to Dr. Basim A. Almayahi, Department of Environment, College of Science, University of Kufa for assisting me throughout conducting the present research.
References
1. Carroll K C, Butel J, Morse S. 2015. Jawetz Melnick and Adelbergs Medical Microbiology 27 E. McGraw-Hill Education. 273-275. Ref.: https://bit.ly/2SqfHBy
2. Pal R R, Baidya AK, Mamou G, et al. 2019. Pathogenic E. coli extracts nutrients from infected host cells utilizing injectisome components. Cell, 177: 683-696. Ref.: https://bit.ly/2SqfHBy
3. Todar K. 2008. Pathogenic E. coli. University of Wisconsim -Madisan Department of Bacteriology.Todar's Online Textbook of Bacteriology.
4. Holt JG, Krieg NR, Sneath Pa, et al. 1994. “Bergey's Manual of Determinative Bacteriology, Ninth Edition, Philadelphia. 273-275. Ref.: https://bit.ly/2JzxmDT
5. Carroll KC, Butel J, Morse S. 2015. Jawetz Melnick and Adelbergs Medical Microbiology 27 E. McGraw-Hill Education. Ref.: https://bit.ly/2SqfHBy
6. Iliyasu MY, Uba A, Agbo EB. 2018. Phenotypic detection of multidrug resistant extended-spectrum beta-lactamase (ESBL) producing Escherichia coli from clinical samples. African Journal of Cellular Pathology. 9:25-32. Ref.: https://bit.ly/30zIFSe
7. Eliakim-Raz N, Babitch T, Shaw E, et al. 2018. Risk factors for treatment failure and mortality among hospitalized patients with complicated urinary tract infection: a multicenter retrospective cohort study (RESCUING Study Group). Clinical Infectious Diseases. 68:29-36. Ref.: https://bit.ly/2XUzolO
8. Japoni A, Vazin A, Hamedi M, et al. 2009. Multidrug-resistant bacteria isolated from intensive-care-unit patient samples. Brazilian Journal of Infectious Diseases. 13: 118-122. Ref.: https://bit.ly/2XBGVqm
9. Hirsch LR, Halas NJ, West JL. 2005. Whole-blood immunoassay facilitated by gold nanoshell-conjugate antibodies. In Nanobiotechnology protocols. 303:101-111. Ref.: https://bit.ly/2Yfz31H
10. Gupta AK, Gupta M. 2005. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials. 26:3995-4021. Ref.: https://bit.ly/2GcibhJ
11. Zeinali Sehrig F, Majidi S, Nikzamir N, et al. 2016. Magnetic nanoparticles as potential candidates for biomedical and biological applications. Artificial cells, nanomedicine, and biotechnology. 44: 918-927. Ref.: https://bit.ly/2XQNipm
12. Fu G, Vary PS, Lin CT. 2005. Anatase TiO2 nanocomposites for antimicrobial coatings. The Journal of Physical Chemistry B. 109: 8889-8898. Ref.: https://bit.ly/2Y7l39U
13. Warheit DB. 2008. How meaningful are the results of nanotoxicity studies in the absence of adequate material characterization? Toxicological sciences, 101:183-185. Ref.:
14. Wilson ML, Gaido L. 2004. Laboratory diagnosis of urinary tract infections in adult patients. Clinical infectious diseases, 38(8), 1150-1158. Ref.: https://bit.ly/30BzC36
15. Ferris RA, Palmer BA, Borlee BR, 2017. Ability of Chromogenic Agar, MALDI-TOF, API 20E and 20 Strep Strips, and BBL Crystal Enteric and Gram-Positive Identification Kits to Precisely Identify Common Equine Uterine Pathogens. Journal of equine veterinary science. 57:35-40. Ref.: https://bit.ly/2JLlD48
16. Jorgensen JH, Turnidge JD. 2015. Susceptibility test methods: dilution and disk diffusion methods. In Manual of Clinical Microbiology. 11:1253-1273. American Society of Microbiology. Ref.: https://bit.ly/2JLLpFh
17. Chou B. 2006. Nano-scale modified inorganic/organic hybrid materials as proton conductors.
18. Zhang Z, Zhang Q, Xu Ln, et al. 2007. Preparation of Nanometer γ?Fe2O3 by an Electrochemical Method in Non?aqueous Medium and Reaction Dynamics. Synthesis and Reactivity in Inorganic, Metal-Organic and Nano-Metal Chemistry. 37: 53-56. Ref.: https://bit.ly/31lsgSF
19. Moloto N, Mpelane S, Sikhwivhilu LM. 2012. Optical and morphological properties of ZnO-and TiO2-derived nanostructures synthesized via a microwave-assisted hydrothermal method. International Journal of Photoenergy. Ref.: https://bit.ly/30B2msK
20. Gupta U, Rudramma, Rati E, et al. 1998. Nutritional quality of lactic fermented bitter gourd and fenugreek leaves. International journal of food sciences and nutrition. 49: 101-108. Ref.: https://bit.ly/2MA00s7
21. Yaseen SS. 2014. Statistical study of urinary tract infection at all in children under the age of five in the city of Kirkuk. Kirkuk Univ. 9: 22-42. Ref.: https://bit.ly/30Hfehf
22. Rayes S, Suhad Faisul Hatim, Ishraq Hassan. 2010. Antimicrobial Susceptibility Patterns of Escherichia coli and Klebsiella pneumoniae among urinary tract infections. Al- Mustansiriya J Sci. 22: 43-50.
23. Al-Attar Zaid I. 2014. The prevalence and antimicrobial sensitivity of Escherichia coli . in clinical isolates. Al-kindy Coll Med J. 10: 96-99. Ref.: https://bit.ly/2XPNdlK
24. Karlowsky JA, Kelly LJ, Thornsberry C, et al. 2002. Trends in antimicrobial resistance among urinary tract infection isolates of Escherichia coli from female outpatients in the United States. Antimicrobial agents and chemotherapy. 46: 2540-2545. Ref.: https://bit.ly/2WyuNtW
25. Rivera-Tapia JA. 2003. Antibiotic resistance, public health problem. An Med Asoc Med Hosp ABC. 48: 42-47. Ref.: https://bit.ly/2F1lQi5
26. Izdebski R, Baraniak A, Fiett J, et al. 2013. Clonal structure, extended-spectrum β-lactamases, and acquired AmpC-type cephalosporinases of Escherichia coli populations colonizing patients in rehabilitation centers in four countries, Antimicrobial agents and chemotherapy. 57: 309-316. Ref.: https://bit.ly/2K97XT9
27. Sasaki T, Hirai I, Niki M, et al. 2010. High prevalence of CTX-M β-lactamase-producing Enterobacteriaceae in stool specimens obtained from healthy individuals in Thailand. Journal of antimicrobial chemotherapy. 65:666-668. Ref.: https://bit.ly/2LTKc1r
28. Bennett JE, Dolin R, Blaser MJ. 2016. Mandell, Douglas and Bennett’s Infectious Disease Essentials E-Book. Elsevier Health Sciences. Ref.: https://bit.ly/2xSdiGm
29. Almayahi BA, Alhusseini LB. 2016. Synthesis and applications of silver nanoparticles on bacterial pathogens activity. Chemtech Research. 9:287-298. Ref.: https://bit.ly/2YRN7eC
30. Hussain DH, Khadam ZA, Marjani MF, et al. 2018. Effect of ZnO Nanoparticles, Fullerene (C60) and Pyocyanin on Imipenem Resistant Gram-Negative Bacteria Isolated from Hospital Environment. Indian Journal of Public Health Research & Development 9: 779-783. Ref.: https://bit.ly/2xQV5sM
31. He S, Feng Y, Gu N, et al. 2011. The effect of γ-Fe2O3 nanoparticles on Escherichia coli genome. Environmental pollution. 159: 3468-3473. Ref.: https://bit.ly/2F0CTke
32. Lin X, Li J, Ma S, et al. 2014. Toxicity of TiO2 nanoparticles to Escherichia coli: effects of particle size, crystal phase and water chemistry. PloS one. 9: 1-8. Ref.: https://bit.ly/2Ze5dqX




















