Indexing & Abstracting
Full Text
Research ArticleDOI Number : 10.36811/ijbm.2019.110016Article Views : 34Article Downloads : 37
In vitro susceptibility testing of Candida species isolated from blood stream infections to five conventional antifungal drugs
Mahdis Hosein Khezri1, Farideh Zaini1*, Parivash Kordbache1, Mohammad Ghahri2, Pegah Ardi1 and Zahra Rafat1
1Department of Medical Parasitology and Mycology, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran
2Health care unit, Tehran, Iran
*Corresponding Author: Professor Farideh Zaini, Department of Medical Parasitology and Mycology, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran, Tel: +98- 21 4293 3150; Fax: +98 21 88951392; Email: Fzaini@tums.ac.ir
Article Information
Aritcle Type: Research Article
Citation: Mahdis Hosein Khezri, Farideh Zaini, Parivash Kordbache, et al. 2019. In vitro susceptibility testing of Candida species isolated from blood stream infections to five conventional antifungal drugs. Int J Biol Med. 1: 124-129.
Copyright: This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Copyright © 2019; Mahdis Hosein Khezri
Publication history:
Received date: 09 October, 2019Accepted date: 16 October, 2019
Published date: 19 October, 2019
Candida is an opportunistic fungal pathogen which can cause fatal bloodstream infections (BSIs) in immunocompromised and immunodeficient persons. In this study, the susceptibility of 196 Candida species isolated from bloodstream infections (BSI) to 5 antifungal drugs were conducted from October 2014 through October 2017. The antifungal drugs used in this study were including fluconazole, itraconazole, voriconazole, Amphotericin B and Caspofungin. From 196 studied isolates, Candida albicans comprised 63.3% of the isolates, followed by C.parapsilosis (18.9 %), C. glabrata (8.7%), C. tropicalis (6.1%), C.krusei (2%) and C.gillermundii (1%). In this study, all isolates of Candida albicans and Candida non-albicans species were completely resistant to voriconazole and itraconazole. Of the 196 Candida isolates, 80 isolates with MIC of 32-16 μg/ml had a dose dependent susceptibility to fluconazole and 111 isolates showed resistance (MIC=64 μg / ml) and only 5 isolates were sensitive (MIC=8 μg / ml) to fluconazole. In this study, out of 196 isolates, 37 isolates were sensitive to amphotericin (MIC=1 μg/ml) and 159 isolates were resistant to amphotericin B (MIC>1 μg/ml). Caspofungin was effective on 104 isolates (MIC<2 μg/ml) and 92 isolates were non-susceptible (MIC>2 μg / ml) to this drug. Out of 124 isolates of Candida albicans, 3 were susceptible, 61 susceptible dose dependent and 60 were resistant to fluconazole. Only 24 isolates were susceptible to amphotericin B and 100 isolates showed resistance to this antifungal drug. Eighty-eight isolates were sensitive to caspofungin and 36 isolates were insensitive. With respect to susceptibility to fluconazole, among 37 isolates of Candida parapsilosis, one was identified as susceptible, 13 isolates were susceptible dose dependent and 13 were resistant. Of these isolates, five were susceptible and 32 isolates were resistant to amphotericin B and caspofungin. Of 12 isolates of Candida tropicalis, 11 showed resistance and 1 was susceptible dose dependent to fluconazole. Of these isolates, 11 were resistant to amphotericin B and 1 isolate was sensitive. Ultimately, only 2 isolates showed susceptibility to caspofungin. Out of 17 isolates of Candida glabrata, 13 isolates were resistant, and 4 isolates had a dose-dependent sensitivity to fluconazole. Eight isolates were susceptible and 9 isolates were resistant to caspofungin. Seven isolates were susceptible and 10 isolates were resistant to amphotericin B. All four Candida krusei isolates showed resistance to the five drugs used in the study. Of the two Candida guilliermondii isolates, both were resistant to amphotericin B, but 1 was sensitive to fluconazole and 1 was identified to be dose-dependent susceptible. One isolate was resistant to and the other one was susceptible to caspofungin. Our findings shows the prevalence of resistant candida species to conventional treatments and indicate that candidemia caused by Candida resistant species is incrasing.
Keywords: Candidiasis; Antifungal drugs; Candidemia; Iran
Introduction
Candida species exist normally in human skin surfaces and mucus membranes. When an imbalance in the normal flora occurs, it causes an overgrowth of Candida species and leads to invasive Candidiasis. Candida bloodstream infection, which is the most common form of invasive candidiasis, is called candidemia [1-3]. Although the mortality rate of candidemia is high, it can decrease with a rapid diagnosis and an effective treatment [4]. In the hospital, 40% of bloodstream infections are caused by the fungus Candida. Most often, Candidemia develops within a week of being admitted to an intensive care unit (ICU) if you also have a central venous catheter, get kidney dialysis, have major surgery, have a low white blood count, or if you are getting broad spectrum antibiotics, intravenous steroids or medications to depress your immune system you can get Candidemia. You can also get Candidemia outside of the hospital if you are sent home from the hospital with a central venous catheter or if you are on cancer chemotherapy. Chemotherapy weakens your immune system and can put you at risk for common infections, like the cold or flu, or less common infections, like Candidemia or other fungal infections. Candida species can also enter the bloodstream alongside a catheter in your vein or artery, at the area where the catheter enters through the skin. Although Candida infections of the mucosal surfaces (mouth and esophagus) are usually easy to treat, treatment of Candidemia can be challenging, especially when the infection has spread to other organs (eye, brain or kidneys) and if there is a central venous catheter in place. By identifying the sensitivity of different species of blood-derived candida to common antifungal drugs such as azoles, polyenes and echinocandins, the mortality rate of candidemia can be reduced [5]. To evaluate the antifungal drug resistance of the etiologic agents of candidemia, we conducted a susceptibility test for Candida species isolated from blood stream infection in Tehran, the center of Iran.
Ethics statement:
This study was approved by ethical committee of Tehran University of medical science.
Sampling and Antifungal Susceptibility Testing:
A total of 196 candida species were used in this study. These species were isolated from blood culture of patients referring to Tehran's hospitals and were collected during 3 years from 2014 to 2017. They were priory identified by PCR Sequencing from the D1-D2 subunit of the 28S recombinant DNA [6]. Antifungal susceptibility testing was performed by broth microdilution with fluconazole, itraconazole, voriconazole, caspofungin and Amphotericin B (soluble in Dimethyl sulfoxide) as described by Clinical and Laboratory Standards Institute (CLSI) M27-A3 guidelines [7] Briefly, testing was performed with RPMI 1640 medium (TREK Diagnostics, Cleveland, Ohio) supplemented with 2% glucose; an inoculum size of 105 CFU/ML was used, as were flat-bottom microdilution plates. MIC endpoints were determined spectrophotometrically at 24 and 48 h. It should be noted that for amphotericin B, the MIC endpoint was defined as the lowest drug concentration that resulted in a reduction in growth of 90% or more, compared with that of a drug-free growth control well. For flucytosine and azoles, the MIC endpoint was defined as a 50% reduction in optical density. For caspofungin, the endpoint was defined according to reports recently published (50% or greater inhibition relative to the control), which analysed influences of methodological variables on susceptibility testing of caspofungin against Candida species [8,9].
Statistical tests
In this study, the Kolmogorov-Smirnov test was used to determine the normal distribution of variables. To test the qualitative variables, chi-square test was used. For quantitative variables and normal distribution, in order to compare the variables in two groups the t-test was used, in abnormal distribution or nonparametric distribution Mean-Whitney test was used. To compare quantitative variables in more than two groups, the parametrical ANOVA test and for no quantitative and abnormal variables Kruskal -Wallis test was used. All data were analyzed using SPSS version 22 software. P value <0.5 considered as meaningful in this study.
Results
In this study, from 196 candida species isolated from blood stream infections relating to patients referring to Tehran city health care centers, Candida albicans with 124 cases (63.3%) was the predominant isolate followed by C.parapsilosis with 37 cases (18.9 %), C. glabrata with 17 cases (8.7%), C. tropicalis with 12 cases (6.1%), C.krusei with 4 cases (2%) and C.gillermundii with 2 cases (1%). (Figure 1).
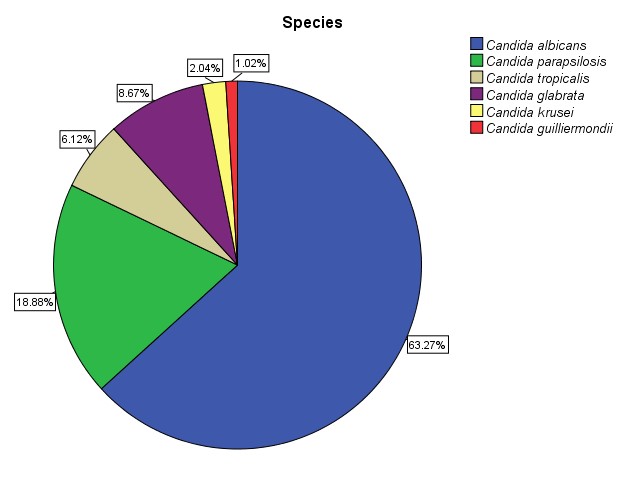
Figure 1: The frequency of candida species isolated from blood stream infections.
This study showed that from 124 C. albicans isolates, 24 isolates were susceptible to Amphotericin B (MIC≤1 μg/ml) and 100 isolates were resistant to this drug (MIC>1 μg/ml). About fluconazole 3 isolates were susceptible (MIC≤8 μg/ml), 61 isolates had dose dependent susceptibility (MIC≤32 μg/ml) and 60 isolates were resistant to this drug (MIC ≥ 64 μg/ml) and about capofungin 88 isolates were susceptible (MIC≤2 μg/ml) and 36 isolates were resistant to this drug. Also in this study, only in one C.albicans isolate dose dependant susceptibility to voriconazole was seen (MIC=2 μg/ml) and all of them were resistant to itraconazole (MIC≥1 μg/ml).
According to Kruskal-Wallis test, there was a significant difference between the MIC values of Candida albicans isolates against amphotericin B (P=0.01), fluconazole (P=0.01), itraconazole (P=0.012), Voriconazole (P=0.028) and capofungin (P=0.002). Also, in this study, from 37 C. parapsilosis isolates, 5 isolates were susceptible to Amphotericin B (MIC≤1 μg/ml) and 32 isolates were resistant to this drug (MIC>1 μg/ml). About fluconazole 1 isolate was susceptible (MIC≤8 μg/ml), 13 isolates had dose dependent susceptibility (MIC≤32 μg/ml) and 23 isolates were resistant to this drug (MIC≥64 μg/ml) and about capofungin 5 isolates were susceptible (MIC≤2 μg/ml) and 32 isolates were resistant to this drug. Also, in this study, and all of studied C. parapsilosis isolates were resistant to itraconazole (MIC≥1 μg/ml) and voriconazole (MIC≥2 μg/ml). According to Kruskal-Wallis test, there was a significant difference between the MIC values of Candida parapsilosis isolates against fluconazole (P=0.0001), itraconazole (P=0.012) , Voriconazole (P=0.002) and capofungin (P=0.004) and there was no significant difference between the MIC values of Candida parapsilosis isolates against amphotericin B (P=0.9).
Also, from 17 C. glabrata isolates, 7 isolates were susceptible to Amphotericin B (MIC≤1 μg/ml) and 10 isolates were resistant to this drug (MIC>1 μg/ml). About fluconazole 4 isolates had dose dependent susceptibility (MIC≤32 μg/ml) and 13 isolates were resistant to this drug (MIC≥64 μg/ml) and about capofungin 8 isolates were susceptible (MIC≤2 μg/ml) and 9 isolates were resistant to this drug Also in this study, and all of studied C.glabrata isolates were resistant to itraconazole (MIC≥1 μg/ml) and voriconazole (MIC≥2 μg/ml). According to Kruskal-Wallis test, there was a significant difference between the MIC values of Candida glabrata isolates against amphotericin B (P=0.01), fluconazole (P=0.00), itraconazole (P=0.012)), Voriconazole (P=0.002) and capofungin (P=0.001). Also from 12 C. tropicalis isolates, 1 isolates were susceptible to Amphotericin B (MIC≤1 μg/ml) and 11 isolates were resistant to this drug (MIC>1 μg/ml). About capofungin 2 isolates were susceptible (MIC≤2 μg/ml) and 10 isolates were resistant to this drug. Also, in this study, and all of studied C. tropicalis isolates were resistant to fluconazole (MIC≥64 μg/ml) itraconazole (MIC≥1 μg/ml) and voriconazole (MIC≥2 μg/ml).
According to Kruskal-Wallis test, there was a significant difference between the MIC values of C. tropicalis isolates against fluconazole (P=0.001), itraconazole (P=0.012), Voriconazole (P=0.002) and capofungin (P=0.002) and there was no significant difference between the MIC values of C. tropicalis isolates against amphotericin B (P=0.7). Also from 4 C.krusei isolates all of them were resistant to all of 5 antifungal drugs used in this study. And from 2 C. gillermundi isolates, both of them were susceptible to fluconazole (MIC≤8 μg/ml) and voriconazole (MIC≤ 2 μg/ml) and about capofungin 88 isolate was susceptible (MIC≤2 μg/ml) and 36 isolates was resistant to this drug. Also, in this study, both of of C. gillermundi isolates were resistant to itraconazole (MIC≥1 μg/ml) and Amphotericin B (MIC>1 μg/ml) (Table 1).
|
Table 1: In vitro susceptibilities to 5 antifungal agents of Candida bloodstream isolates. |
||||||||||||||||
|
Antifungal agents |
Caspofungin |
AmphotericinB |
voriconazole |
itraconazole |
fluconazole |
|||||||||||
|
Candida species |
R |
SDD |
S |
R |
SDD |
S |
R |
SDD |
S |
R |
SDD |
S |
R |
SDD |
S |
|
|
C. alb |
36 |
-- |
88 |
100 |
-- |
24 |
124 |
0 |
0 |
124 |
0 |
0 |
60 |
61 |
3 |
|
|
C.parapsilosis |
32 |
-- |
5 |
32 |
-- |
5 |
37 |
0 |
0 |
37 |
0 |
0 |
23 |
13 |
1 |
|
|
C.glabrata |
9 |
-- |
8 |
10 |
-- |
7 |
17 |
0 |
0 |
17 |
0 |
0 |
13 |
4 |
0 |
|
|
C.tropicalis |
10 |
-- |
2 |
11 |
-- |
1 |
12 |
0 |
0 |
12 |
0 |
0 |
11 |
1 |
0 |
|
|
C.krusei |
4 |
-- |
0 |
4 |
-- |
0 |
4 |
0 |
0 |
4 |
0 |
0 |
4 |
0 |
0 |
|
|
C.gillermundi |
1 |
-- |
1 |
2 |
-- |
0 |
2 |
0 |
0 |
2 |
0 |
0 |
0 |
1 |
1 |
|
Discussion
Candida species are the most common cause of systemic fungal infections and globally, out of 1,000 patients, 6.9% are suffering from candidiasis [10,11]. In recent years, a significant increase in the incidence of candidemia has been observed. Candida species are reported as one of the five most common causes of sepsis in the United States and one of 10 most common causes of sepsis in Europe. In hospitals, up to 40% of the blood infections could be related to Candida species [12-14]. This increase could be duo to the increased number of patients with immune deficiency, AIDS, solid organ or bone marrow transplantation, and intrinsic and acquired resistance of Candida species to antifungal drugs. Although candidemia-related mortality is high, it could be decreased with timely diagnosis of the disease and the early onset of treatment. Determination of susceptibility pattern for blood-derived Candida isolates to common antifungal drugs, could be beneficial in reducing the mortality rate of candidemia [3]. In this study, all of the Candida species isolated from blood were resistant to itraconazole and voriconazole. This is due to the increased use of these drugs and the alteration of the cellular structure of Candida albicans and non-albicans.
These results are consistent with previous studies in this field [15,17]. Also, this study showed that caspofungin was more effective on Candida albicans isolates and Amphotricin B had the most effective on Candida albicans isolates. This funding is consist with the study published with previous studies in this field [16,17]. The results of this study indicate that candidemia caused by Candida resistant species, especially occurs in patients with underlying and predisposing diseases. Therefore, considering the diagnostic problems and adverse effects of candidiasis in these patients, further research is recommended in this regard.
Acknowledgments
The authors appreciate the support of Tehran University of Medical Sciences, Tehran, Iran.
References
1. Somerville DA. 1969. The normal flora of the skin in different age groups. British Journal of Dermatology. 81: 248-258. Ref.: https://bit.ly/2ONEUWM
2. RAFAT Z, HASHEMI S, AHAMDIKIA K, et al. 2017. Study of skin and nail Candida species as a normal flora based on age groups in healthy persons in Tehran-Iran. Journal de mycologie medicale. 27: 501-505. Ref.: https://bit.ly/2B92ZPP
3. Mikulska M, Del Bono V, Ratto S, et al. 2012. Occurrence, Presentation and Treatment of Candidemia. Expert Rev Clin Immunol. 8: 755-765. Ref.: https://bit.ly/2VI33iM
4. Odds FC, Schmid J, Soll DR. 1990. Epidemiology of Candida infection in AIDS. Plenum Press, New York, N.Y). 67-74.
5. Cleveland AA, Farley MM, Harrison LH, et al. 2012. Changes in incidence and antifungal drug resistance in candidemia: results from population-based laboratory surveillance in Atlanta and Baltimore, 2008-2011. Clinical infectious diseases. 55: 1352-1361. Ref.: https://bit.ly/2qafCrq
6. Deak E, Etienne KA, Lockhart SR, et al. 2010. Utility of a Luminex-based assay for multiplexed, rapid species identification of Candida isolates from an ongoing candidemia surveillance, Can J Microbiol. 56: 348-351. Ref.: https://bit.ly/2MhzscW
7. Institute CaLSM27-A3 Reference method for broth dilution antifungal susceptibility testing of yeasts: approved standard-third edition, 2008Wayne, PAClinical Laboratory Standards Institute.
8. Odds FC, Motyl M, Andrade R. et al. 2004. Interlaboratory comparison of results of susceptibility testing with caspofungin against Candida and Aspergillus species. Journal of Clinical Microbiology. 42: 3475-3482. Ref.: https://bit.ly/31d4R4E
9. Bartizal C, Odds FC. 2003. Influences of methodological variables on susceptibility testing of caspofungin against Candida species and Aspergillus fumigatus. Antimicrobial Agents and Chemotherapy. 47: 2100-2107. Ref.: https://bit.ly/2VI1sJI
10. Bouza E, Munoz P. 2008. Epidemiology of candidemia in intensive care units. Int. J. Antimicrob. Agents. 32: 87-91. Ref.: https://bit.ly/2IQpBc4
11. Bassetti M, Taramasso L, Nicco E, et al. 2011. Epidemiology, species distribution, antifungal susceptibility and outcome of nosocomial candidemia in tertiary care hospital in Italy. PLoS ONE. 2011: 6. Ref.: https://bit.ly/31c4CGN
12. Martin GS, Mannino DM, Eaton S, et al. 2003. The epidemiology of sepsis in the United States from 1979-2000. N Engl J Med. 348: 1546-1554. Ref.: https://bit.ly/33xKAs6
13. Quindos G. 2014. Epidemiology of candidemia and invasive candidiasis. A changing face. Rev Iberoam Micol. 31: 42-48. Ref.: https://bit.ly/2VIo90t
14. Antinori S, Milazzo L, Sollima S, et al. 2016. Candidemia and invasive candidiasis in adults: A narrative review. Eur J Intern Med. 34: 21-28. Ref.: https://bit.ly/33y2KtO
15. Sobel JD. 1999. Management of asymptomatic candiduria. International journal of antimicrobial agents. 11: 285-288. Ref.: https://bit.ly/2pgZowh
16. Haddadi P, Zareifar S, Badiee P, et al. 2014. Yeast colonization and drug susceptibility pattern in the pediatric patients with neutropenia. Jundishapur Journal of Microbiology. 7: 1-6. Ref.: https://bit.ly/2BovoS9
17. Elham Baghdadi, Sadegh Khodavaisy, Sassan Rezaie, et al. 2016. Antifungal Susceptibility Patterns of Candida Species Recovered from Endotracheal Tube in an Intensive Care Unit. 6. Ref.: https://bit.ly/2IRMXOB




















