Indexing & Abstracting
Full Text
Research ArticleDOI Number : 10.36811/ijho.2019.110002Article Views : 52Article Downloads : 43
Effect of Green Tea and Zinc oxide Nanoparticles Complex on Histopathology of Spleen of Male Rats Induced by Monosodium Glutamate
Fawziah A Al-Salmi1, Reham Z Hamza1,2 and Nahla S El-Shenawy3
1Biology Department, Faculty of Science, Taif University, Taif, Saudi Arabia
2Zoology Department, Faculty of Science, Zagazig University, Zagazig, Egypt
3Zoology Department, Faculty of Science, Suez Canal University, Ismailia, 41522, Egypt
*Corresponding Author: NS El-Shenawy, Prof. of Physiology and Toxicology, Zoology Department, Faculty of Science, Suez Canal University, Ismailia 41522, Egypt, Tel: 002/01008660620; Email: elshenawy_nahla@hotmail.com; nahla.el-shennawy@science.scu.egypt.edu.eg
Article Information
Aritcle Type: Research Article
Citation: Fawziah A Al-Salmi, Reham Z Hamza, Nahla S El-Shenawy. 2019. Effect of Green Tea and Zinc oxide Nanoparticles Complex on Histopathology of Spleen of Male Rats Induced by Monosodium Glutamate. Int J Hematol Oncol. 2: 04-11.
Copyright: This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Copyright © 2019; Fawziah A Al-Salmi
Publication history:
Received date: 10 August, 2019Accepted date: 27 August, 2019
Published date: 30 August, 2019
Abstract
Background and objective: Synthesis of zinc oxide nanoparticles (ZnO NPs) with green tea extract (GTE) to form a complex is known to be one of the most multiuse nanoparticles with its application in treatment the toxicity of monosodium glutamate (MSG) on liver, kidney, testis, and pancreas. Therefore, the present study was concerned with the pontifical effect of ZnO NPs / GTE complex on the histological structure of spleen exposed to MSG.
Materials and Methods: The toxicity of MSG was evaluated in male albino rats using two dosages (low, 6.0 and high, 17.5 mg/kg). The albino rats were taken for the experiment and randomly assigned into six groups; control, ZnO NPs, MSG-LD, MSG-HD, ZnO NPs / GTE + MSG-LD, and ZnO NPs / GTE + MSG-HD. The animals were decapitated after 30 days of exposure and spleens were dissected out and processed for the histological examination by light microscope.
Results: The result revealed that MSG causes shrinkage in the white pulp nodule with increasing the area of the white pulp and degeneration of red pulp as compared to the control. The changes were more prominent in the rats treated with the higher dosage of MSG. The finding suggests that MSG may effect on adhesion of splenocytes and degeneration of red pulp in the rat leading to the reduced immunogenic response.
Conclusions: The data could be demonstrated the effect of MSG on spleen tissue was a dose-dependent and led to hypertrophy of white pulp of the spleen. The ZnO NPs/GTE complex could provide a protective benefit against MSG-induced splenomegaly through its potent antioxidant properties due to the presence of GTE and reduction of the ZnO. The future study will be a concern on the thymus histology as it acts as the center of lymphoid organ.
Keywords: Monosodium glutamate, ZnO nanoparticles, Spleen, Histology, Rats
Introduction
Monosodium glutamate (MSG is the glutamic acid core sodium salt that is easily soluble in water. It is a food additive that has trade names as Ajinomoto, Chinese salt, and E621 in many nations. It is widely used in many foods like chips, noodles, and soups to create a unique color or taste and attract customers, particularly kids [1]. Several previous studies have shown that MSG associated with many pathological circumstances such as depression, Alzheimer's disease, epilepsy, liver diseases, and kidney disorder [2,3]. MSG has been shown to cause its toxicity by inducing oxidative stress in the different organs such as brain [4], spleen [5] pancreas [6], liver [7], kidney [8] and thymus [9] as well as through necrosis and apoptosis. ZnO nanoparticles are among the nanoparticles that are most toxic. They promote the development of reactive oxygen species (ROS) that interfere with biochemical intracellular activity and antioxidant mechanism. These alternations allow for the interaction and harm of freshly produced ROS with lipids, carbohydrates, proteins, and DNA [10]. Moreover, they are soluble to generate cytotoxic impacts, oxidative stress, and mitochondrial dysfunction at levels elevated enough [11-13]. They are readily taken into the bloodstream by the gastrointestinal system when it was administrated as a single oral dose. It can be accumulated in the body causing poisonous depending on the particle size and age. Nanoparticles were synthesized as nontoxic and environmentally friendly reduction material using green tea leaf extract, leading to genuinely green chemistry that is also efficient at very inexpensive costs, as elevated stress, energy, temperature, and toxic chemicals are not required. For pharmaceutical and other biomedical products, ZnO nanoparticles provide countless benefits of Eco friendliness and compatibility [14].
Moreover, biosynthesis of ZnO NPs using green tea leaves extracts (GTE) was taken very short-time, using economical equipment and produces a pure product free of contamination [11-13]. The synthesized ZnO NPs/GTE were characterized by using a scanning electron microscope. The C, H, Zn examination comes about of the yttrium complex appeared near closeness to the hypothetical values. The results of their elemental analysis have relegated the atomic formulae of the complex. The elemental analysis of the complex yielded 40.77 % of zinc, 30.18% of oxygen and 29.05% of carbon which proves that the produced ZnO NPs complex with GTE is in its highest purified formula and confirmed it's a combination [14]. This complex (ZnO NPs/GTE) has been proved that it had a protective effect against MSG-induced in the liver, kidney, and brain [7,11,12,14].
The spleen in the left cranial abdomen is a pale red to blue-black organ. It is an elongated organ, in the cross-section approximately triangular. It is the biggest secondary lymphoid immune organ with approximately one-fourth of the lymphocytes in the body. It is consists of two distinct functional and morphological compartments, the red pulp and the white pulp [15]. The spleen's functions focus on the systemic circulation It is responsible for initiating immune reactions to blood-borne antigens and for filtering the blood of foreign material and old or damaged erythrocytes as well as it is an iron, red blood cells and platelets disposal location. These functions are carried out by the 2 main compartments of the spleen, the white pulp (including the marginal zone) and the red pulp, which are vastly different in their architecture, vascular organization, and cellular composition [16]. The spleen is the locations of immediate and indirect poisoning and a base for certain carcinogens, as well as metastasis locations for malignant neoplasms in others. Following our previous research work on green tea and ZnO nanoparticles complex effects on different organs (liver, kidney, brain, and pancreas), the current study explains the effect of ZnO NPs/GTE complex on histopathology of rats’ spleen induced by MSG.
Materials and Methods
Chemicals
The Green tea plant with high purity was obtained from the local market of Al-Taif city – Saudi Arabia. The ZnO NPs with a diameter of 200 nm, length up to 150 nm and size range 40-150 nm were obtained from Sigma-Aldrich Company. The purities of ZnO NPs were 99.5 % Sigma Aldrich. Monosodium glutamate (MSG) with purity 99% was obtained from markets (Ajinomoto Co. Inc., Tokyo, Japan).
Preparation of green tea extracts (GTE), MSG and ZnO NPs/GTE complex
An amount of 10 g of finely green tea was mixed with 100 mL ethanol 95º in 35 ºC. The mixed solution was left on persistent magnetic stirring for 24 hours at 27 ºC [17]. The green tea extract (GTE) was filtered and placed for further studies at 4?C. The ZnO NPs with a diameter of 200 nm, length up to 150 nm, size range 40-150 and purity 99.5 % were obtained from Sigma-Aldrich Company. The 0.001 M aqueous solutions of ZnO NPs were prepared by dispersing using ultrasonic vibration (130 W, 20 kHz) for 30 min and used for the synthesis of ZnO NPs / green tea complex [11].
120 mL aqueous solution of 0.001 M ZnO NPs and 5 mL of leaves green tea extract (GTE) were mixed and kept at room temperature for 3-4 hrs for the reduction process of Zn ions. Finally, the GTE / ZnO NPs complex (1 mg/mL) was formed. Monosodium glutamate (C5H9NO4·Na, MSG) was obtained from open markets under the license of Ajinomoto Co. The stock solution was prepared by dissolving of 60 g of MSG crystals in 1000 mL of distilled water.
Animals and experimental design
The Wistar rats were purchased from the animal house of the Faculty of Pharmacy – Zagazig University, Egypt. Forty-eight adult male rats were weight 200-250 g and kept under standard laboratory conditions for two weeks before being experimented. The animals were allowed to food and water throughout the experimental period. The Research Animal Ethics Committee at Taif University approved the protocol of animal care (39-31-0034). The rats were assigned to six groups (n=8). Control group was given the physiological saline solution. The other five groups treated orally for 30 days as following: ZnO NPs / GTE complex (1mg/mL), MSG-LD (6 mg/Kg), MSG-HD (17.5 mg/Kg), MSG-LD + ZnO NPs/GTE complex and MSG-HD + ZnO NPs/GTE complex. The applied treatments dosages of MSG were selected according to the previous study of Hamza and Al-Harbi [18] while the dose of ZnO NPs was selected depend on Ben-Slama et al [19]. The Research Animal Ethics Committee at Taif University approved our standards of animal care (39-31-0034) and considered that they are consistent with the requirements and standards of international laws and regulations of the European Community Directive (86/609/EEC).
Histopathological Investigation
Spleen specimens were collected from rats of all experimental groups at the end of the experimental period. The tissue pieces were washed well after fixation in 10% neutral buffered formalin (pH=7.0), dehydrated through a graded series of ethyl alcohol, cleared in xylene and embedded in paraffin wax. 4-5 μm thick sections were cut and stained with hematoxylin-eosin (H-E). Microscopic slides were observed under a light microscope at various magnification and subsequently photographed.
Results
The size of spleen did not change after 30 days of treatment in all group except in the group of rats that treated with a higher dosage of MSG (Figures 1A and 1B).
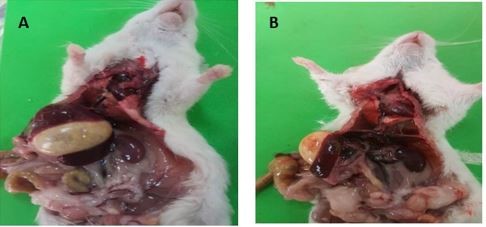
Figure 1: The spleen for control and MSG-HD treated-rats for 30 days (A and B, respectively). The spleen in B with fatty body and splenomegaly (inflammatory, congestive states and hyperplastic).
The structure of control and ZnO NPs/GTE spleen was composed of white and red pulps surrounded by a capsule of dense connective tissue (Figures 2A and 2B). The splenic white pulp was composed of three compartments, where either T or B lymphocytes predominate. The central T-cell zone is called the periarteriolar lymphatic sheath (PALS). The PALS surrounded by other two compartments that are B lymphocytes and the marginal zone. The white pulp was separated from the red pulp by the marginal zone lymphocytes. The lymphatic follicle (LF) with pale staining germinal center was observed. A marginal zone (MZ) is clearly differentiated from the red pulp. Many blood cells are also recognized in the red pulp A full-blown germinal center comprises two areas, the dark, and the light areas. The dark zone is oriented towards the PALS and proliferating B-cells with sparse cytoplasm and large nuclei. The light zone, oriented towards the red pulp, is populated by centrocytes. These cells have smaller nuclei, more cytoplasm, and are less densely arranged than centroblasts. They are supposed to be no dividing differentiating B- lymphocytes arising from centroblasts, which are on their way to becoming plasma cells or memory B cells.
The PALS and the follicles are surrounded by the MZ, which separates both compartments from the splenic red pulp. The MZ is a broad region primarily occupied by relatively large memory B cells. It gives a light impression after routine staining because memory B cells have paler nuclei and more cytoplasm than the T cells of the PALS or the B cells of primary follicles. The MZ is delimited from the PALS and the follicles by a very irregular capillary blood vessel called the marginal sinus. The marginal zone is a unique region of the spleen situated at the interface of the red pulp with the PALS and follicles After 30 days of MSG-LD exposure, the relative area of the marginal zone of lymph follicles was increased as compared to the control rats (Figure 2C). This area increases by increasing the dose of MSG (Figure 2D). The marked dilated and congested splenic sinusoids with degenerated lymphoid cells were frequently seen. The spleen of MSG-treated rat with the higher dosage showed small-sized lymphatic follicles with no germinal centers and large congested blood vessel. Many lymphocytes with different sizes and darkly stained nuclei are seen in the white pulp and blood cells. Splenic sinusoids are scattered in the red pulp. The rats treated with a lower dosage of MSG and ZnO NPs/GTE showed slight atrophy in the white pulp and aplasia of red pulp where the marginal zone between white pulp and red pulp were difficult definite (Figure 2E). The animals that treated with MSG-HD and ZnO NPs/GTE showed vacuolation of some splenic cells with decreased cellularity, and sinusoidal spaces were large (Figure 2F). Many blood cells were present within dilated blood sinusoids.

Figure 2: (A) The spleen of 1st control rat (low magnification) X 40.
(B): Photomicrograph of ZnO NPs/GTE rat spleen showing normal morphology of red pulp with splenic
cords that central pale reactive germinal, peripherally dark stained mantle zone X 100.
(C) Photomicrograph of MSG treated-rat spleen with A lower dosage (6 mg/kg body weight) showing the red pulp X 100.
(D): Photomicrograph of MSG-treated rat spleen with a high dose (17.5 mg/kg-bodyweight) showing degenerated splenic cords and many apoptotic cells in the red pulp with marked congestion X 40. The white pulp attenuated and showing areas of hemorrhage and hemosiderin-laden macrophages X 40.
(E): the spleen of MSG-LD + ZnO NPs showing reactive germinal centers.
(F): MSG-HD +ZnONPs showing reactive germinal centers showing centroblasts, centrocytes, and tangible body macrophages X 100. (H-E stain). (Images scale bar, A,D,E; 1mm & B, C, F; 100 μm). LF – lymphoid follicle; PALS – periarterial lymphoid sheath; RP – red pulp; WP – white pulp; MZmarginal zone; BV-blood vessel; V-vacuole; G- germinal centers are prominent.
Discussion
Treatment the rats with MSG for 30 days induced oxidative stress, inflammation, and histopathological changes in the spleen depending on the dosage of MSG. The green tea leaf extract was used to reduce ZnO NPs toxicity as well as to stabilize them. The reaction occurred at room temperature within 3-4 hours. This also provided powerful proof for the participation of tea leaf polyphenol content in fast biosynthesis and the stabilization of metal nanoparticles in the aqueous medium. ZnO NPs can be achieved by reduction of Zn+2 with the use of green tea leaf that contains epigallocatechin gallate (EGCG), which acts as both the reducing and capping agent. EGCG is a highly water-soluble and highly polarized compound. As such, the EGCG structure provided sufficient reducibility to convert Zn2+ ions into Zn(0) nanoparticles [20]. EGCG is the primary focus for the activity behind green tea consumption. EGCG can inhibit the inflammation in the liver, causing a decrease in oxidative and increase of antioxidant markers in rat liver [7,11], kidney [13], brain, pancreas, and testis [unpublished data]. It is well known that MSG has a disruptive effect on various tissues in the body [2-9], [11,13]. The present results revealed that animals treated with MSG showing cellular disruption and degeneration of the white pulp in the spleen as compared to the control sections. This result is in agreement with Ebaid and Tag [21] that the administration of MSG-induced degenerative and atrophic changes in the rat spleen. Our present finding that there is a decrease in the white pulp with a concomitant increase in red pulp infiltrated by lymphocytes is in agreement with other findings [22]. They studied the effect of sodium fluoride on the spleen. Moreover, the faint eosinophilic staining region observed in the red pulp could be caused by splenocytes apoptosis in MSG-HD treated-rats as well as the degeneration of the extracellular matrix to which the splenocytes have connected. In the MSG-HD treated group, spleen showed small-sized lymphatic follicles with the absence of germinal centers. These results are in agreement with [23] who found that the administration of MSG caused degenerative and atrophic changes in the rat spleen. Tetra-hydroxybutylimidazole is one of additive in food color, decreased the number of splenic lymphocytes, and lymph nodes of rats. Its immunosuppression is believed to inhibit the peripheral motion of mature thymocytes [24] that could happen in MSG-treated rats.
In the pathogenesis of MSG-induced illness, glutamate receptors play a very significant role [24]. T lymphocytes display various kinds of glutamate receptors that regulate the immune responses, activation of the cell, and death [25]. Glutamate modifications the voltage-gated potassium channels function resulting in a rise of intracellular calcium content [26]. Increased calcium flow contributes to unnecessary calcium intake into mitochondria, which can trigger cell death through processes such as the discharge of proapoptotic variables and enhanced ROS production [27]. It is possible that the matrix had undergone degradation resulting in signaling to the cells adhered with it and causing apoptosis. The relative area of marginal zone and relative area of germinal centers of lymph follicles have increased. Our data coincide with the results of another study, who observed hyperplasia of lymphoid tissue following exposure to a certain toxic substance [28]. The administration of ZnO NPs/ GTE with MSG has improved the prior pathological modifications in this present work against MSG-induced splenomegaly through its potent antioxidant properties due to the presence of GTE. Then, frequently adding ZnO NPs/ GTE to meals and restricting the consumption of products containing MSG had been suggested. Further trials are required to determine the efficient dose of ZnO NPs/GTE, which will restore the amount of both T helper and macrophages. Based on these data, food manufacturing organization should take heed and possibly decrease the frequency and level of MSG added to food products. The people should limit their national consumption of foods comprising this flavor enhancer and cautions should be taken in its use in the industries.
Conclusion
In conclusion, MSG has resulted in spleen modification particularly due to apoptosis of splenocytes and degeneration of red pulp in the rat. This may result in reduced immunogenic response for the human beings that used MSG. These changes are probably to be improved by ZnO NPs/ GTE administration.
Significance statement
This study discovered that MSG-induced splenomegaly. MSG may effect on adhesion of splenocytes and degeneration of red pulp. The ZnO NPs / GTE complex was proved to be a potential against hyperplasia of the white pulp. This study will help the researchers to uncover the critical areas of nanoparticle complex that many researchers were not able to explore. Thus, a new theory on beneficial of nanoparticle complex on spleen may be arrived at.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
1. Husarova V and D Ostatnikova. 2013. Monosodium glutamate toxic effects and their implications for human intake: A review. JMED Research Article. Ref.: https://bit.ly/2zn7xB1
2. Narayanan SN, Kumar RS, Paval J, et al. 2010. Effect of ascorbic acid on the monosodium glutamate-induced neurobehavioral changes in preadolescent rats. Bratisl Lek Listy. 111: 247-252. Ref.: https://bit.ly/2ZebSWy
3. Mattson MP. 2008. Glutamate and neurotrophic factors in neuronal plasticity and disease. Ann N Y Acad Sci. 1144: 97-112. Ref.: https://bit.ly/2U4AdZf
4. Onaolapo OJ, Onaolapo AY, Akanmu MA, et al. 2016. Evidence of alterations in brain structure and antioxidant status following 'low-dose' monosodium glutamate ingestion. Pathophysiol. 23: 147-156. Ref.: https://bit.ly/2KWgt6Y
5. Hassan ZA, Arafa MH, Soliman WI, et al. 2014. The effects of monosodium glutamate on thymic and splenic immune functions and role of recovery (biochemical and histological study). J Cytol Histol 5: 283. Ref.: https://bit.ly/2Hse8Pb
6. Nwaopara AO, Anyanwu LC, Oyinbo CA, et al. 2004. Histological changes in pancreas of Wistar rats fed diets containing Yaji (local meat sauce). J Exp Clin Anat. 3: 44- 47.
7. Hamza R, Al-Salm FA, and El-Shenawy NS. 2018. Nanoparticles Effects on Zinc Oxide/green Tea Complex on the Lipid Profile and Liver Functions of Rats after Monosodium Glutamate Treatment J Appl Sci. 18: 65-70. Re.: https://bit.ly/2MGVd7s
8. Nwaopara AO, Odike MA, Inegbenebor U, et al. 2008. A comparative study on effects of excessive consumption of ginger, clove, red pepper, and black pepper on histology of the kidney. Pakistan J Nutr. 7: 287-291. Ref.: https://bit.ly/2KZkvvi
9. Pavlovic V, Pavlovic D, Kocic G, et al. 2007. Effect of monosodium glutamate on oxidative stress and apoptosis in rat thymus. Mol Cell Biochem. 303: 161-166. Ref.: https://bit.ly/2HuiKnT
10. Yin H, Casey PS, and MJ McCall. 2010. Surface modifications of ZnO nanoparticles and their cytotoxicity. J Nanosci Nanotechnol. 10: 7565-7570. Ref.: https://bit.ly/2NAlJyQ
11. Al-Salmi FA, Hamza RZ, El-Shenawy NS. 2019. The interaction of zinc oxide/green tea extract complex nanoparticles and monosodium glutamate in liver of rats. Current Pharm Biotech. 20: 45-475. Ref.: https://bit.ly/2Zh6nGJ
12. El-Shenawy NS Hamza RZ, Al-Salmi FA. 2019. Evaluation of nanoparticles zinc oxide/ Camellia sinensis complex and monosodium glutamate in the kidney of rats: antioxidant and histological approaches Curr Pharmace Biotechnol. In Press. Ref.: https://bit.ly/2Zx6ixT
13. Agarwal H, Kumar SV, Rajeshkumar S. 2017. A review on green synthesis of zinc oxide nanoparticles-An eco-friendly approach. Resource-Efficient Technology. 3: 406-413. Ref.: https://bit.ly/2Hu7Ezd
14. Hamza RZ, Al-Salmi FA, and El-Shenawy NS. 2019. Evaluation of the perpetrates of the green nanoparticles zinc oxide on monosodium glutamate-induced toxicity in the brain of Rats. ASN Neuro. In Press. Ref.: https://bit.ly/32bqYt7
15. Voloshin VN, Koveshnikov VG, and IS Voloshina. 2014. Morphology of the spleen in adult albino rats after whole-body exposure to low-level of toluene. Int J Anat. Res. 2: 421-430. Ref.: https://bit.ly/30A2CZG
16. Cesta MF. 2006. Normal structure, function, and histology of the spleen. Toxicologic Pathol. 34: 455-465. Ref.: https://bit.ly/2MMn8TJ
17. Huang T, Barclay BJ, Kalman TI, et al. 1992. The phenotype of a dihydrofolate reductase mutant of Saccharomyces cerevisiae. Gene. 121:167-171. Ref.: http://tiny.cc/tvnsbz
18. Hamza RZ and MS Al-Harbi. 2014. Monosodium glutamate-induced testicular toxicity and the possible ameliorative role of vitamin E or selenium in male rats. Toxicol Rep. 22:1037-1045. Ref.: http://tiny.cc/kznsbz
19. Ben-Slama I, Mrad I, Rihane N, et al 2015. sub-acute oral toxicity of zinc oxide nanoparticles in male rats. J Nanomed Nanotechnol. 6: 284. Ref.: http://tiny.cc/i3nsbz
20. Liu H, Dong X, Liu F, et al. 2017. Iminodiacetic acid-conjugated nanoparticles as a bifunctional modulator against Zn2+-mediated amyloid β-protein aggregation and cytotoxicity. J. Colloid Interface Sci. 505: 973-982. Ref.: http://tiny.cc/46nsbz
21. Ebaid HM and HM Tag. 2012. Monosodium glutamate toxic effect on spleen structure and potentiality of recovery in adult albino rats. Egypt. Acad J Biolog Sci. 4: 1-8. Ref.: http://tiny.cc/w8nsbz
22. Podder S, Chattopadhyay A, Bhattacharya S, et al. 2010. Histopathology and cell cycle alteration in the spleen of mice from low and high doses of sodium fluoride. Fluoride. 43: 237-245. Ref.: http://tiny.cc/abosbz
23. Ciric M, Cekic S, Pavlovic V, et al. 2005. Histopathological changes In spleen of rats treated with monosodium glutamate. Acta Fac Med. Naiss. 22:191-194. Ref.: http://tiny.cc/9cosbz
24. Greenwood SM and CN Connolly. 2007. Dendritic and mitochondrial changes during glutamate excitotoxicity. Neuropharmacol. 53: 891-898. Ref.: http://tiny.cc/aeosbz
25. Kvaratskhelia E, Maisuradze E, Dabrundashvili NG, et al. 2009. N-methyl-D-aspartate and sigma-ligands change the production of interleukins 8 and 10 in lymphocytes through modulation of the NMDA glutamate receptor. Neuroimmunomodula. 16: 201- 207. Ref.: http://tiny.cc/xfosbz
26. Miglio G, Varsaldi F, and G. Lombardi. 2005. Human T lymphocytes express N-methyl-D-aspartate receptors functionally active in controlling T cell activation. Biochem Biophys Res. Commun. 338: 1875-1883. Ref.: http://tiny.cc/9hosbz
27. Luetjens CM, Bui NT and B Sengpiel. 2000. Delayed mitochondrial dysfunction in excitotoxic neuron death: cytochrome c release and a secondary increase in superoxide production. J Neurosci. 20: 5715-5723. Ref.: http://tiny.cc/nkosbz
28. Frouin H, Fortier M, and M Fournier. 2010. Toxic effects of various pollutants in 11B7501 lymphoma B cell line from harbour seal (Phoca vitulina). Toxicol. 270: 66-76. Ref.: http://tiny.cc/8oosbz




















