Indexing & Abstracting
Full Text
Research ArticleDOI Number : 10.36811/ijho.2020.110004Article Views : 0Article Downloads : 0
Pharmacoeconomics of bortezomib - a cochin cancer research centre evaluation and review
Prem Ravi Varma PK1*, Sinimol VG2, Siji P Jose3, Nikhil Ashok4 and Abhilash AS5
1Headof Department, Department of Medical Oncology, Cochin Cancer Research Centre, India
2Sinimol VG, Department of Pharmacy, Cochin Cancer Research Centre, India
3Siji P Jose, Department of Pharmacy, Cochin Cancer Research Centre, India
4Nikhil Ashok, Department of Biomedical Engineering, Cochin Cancer Research Centre Abhilash AS, India
5Department of Administration, Cochin Cancer Research Centre, India
*Corresponding Author: Dr P K Prem Ravi Varma, Head of Department, Department of Medical Oncology, Cochin Cancer Research Centre, India, Email: narniat@yahoo.com
Article Information
Aritcle Type: Research Article
Citation: Prem Ravi Varma PK, Sinimol VG, Siji P Jose, et al. 2020. Pharmacoeconomics of bortezomib - a cochin cancer research centre evaluation and review. Int J Hematol Oncol. 3: 08-17.
Copyright: This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Copyright © 2020; Prem Ravi Varma PK
Publication history:
Received date: 20 March, 2020Accepted date: 06 April, 2020
Published date: 07 April, 2020
Abstract
Bortezomib also known as Velcade is indicated as a parenteral agent in the treatment of multiple myeloma. In noncomparative trials in multiple myeloma patient’s early treatment was associated with a greater rate of success. Treatment with Bortezomib-based regimens was associated with improvements in health-related quality of life. Orthopaedic surgery for spinal cord compression in patients with multiple myeloma was generally predicted to be cost saving, relative to not having surgery, over the medium to long term in modelled cost analyses from a healthcare payer perspective in the UK and US. The initial cost of surgery was high, but the difference in costs between patients undergoing or not undergoing surgery was predicted to decline over time, as savings were realised from the decrease in the number of thrombocytopaenic episodes requiring treatment in patients who underwent surgery. Available pharmacoeconomic data from several countries including the Cochin Cancer Research Centre in Kerala State, India despite inherent limitations, support the use of Bortezomib in the first line treatment of multiple myeloma.
Introduction
Multiple myeloma (MM), also known as plasma cell myeloma, is a cancer of plasma cells, a type of white blood cell that normally produces antibodies [1]. Often, no symptoms are noticed initially [2]. As it progresses, bone pain, bleeding, frequent infections, and anemia may occur [2]. Complications may include amyloidosis [3]. The cause of multiple myeloma is unknown [4]. Risk factors include obesity, radiation exposure, family history, and certain chemicals [5-7]. Multiple myeloma may develop from monoclonal gammopathy of undetermined significance that progresses to smoldering multiple myeloma [8]. The abnormal plasma cells produce abnormal antibodies, which can cause kidney problems and overly thick blood [2]. The plasma cells can also form a mass in the bone marrow or soft tissue [9]. When one tumor is present, it is called a plasmacytoma; more than one is called multiple myeloma [2]. Multiple myeloma is diagnosed based on blood or urine tests finding abnormal antibodies, bone marrow biopsy finding cancerous plasma cells, and l imaging finding bone lesions [1]. Another common finding is high blood calcium levels [1]. Multiple myeloma is considered treatable, but generally incurable [3]. Remissions may be brought about with steroids, chemotherapy, targeted therapy, and stem cell transplant [3]. Bisphosphonates and radiation therapy are sometimes used to reduce pain from bone lesions [1,3]. Globally, multiple myeloma affected 488,000 people and resulted in 101,100 deaths in 2015 [10,11]. In the United States, it develops in 6.5 per 100,000 people per year and 0.7% of people are affected at some point in their lives [12]. It usually occurs around the age of 61 and is more common in men than women [1]. It is uncommon before the age of 40 [1]. Without treatment, typical survival is seven months [3]. With current treatments, survival is usually 4-5 years [3]. The five-year survival rate is about 49% [12]. The word myeloma is from the Greek myelo- meaning "marrow" and -oma meaning "tumor" [13]. Bortezomib, sold under the brand name Velcade among others, is an anti-cancer medication used to treat multiple myeloma and mantle cell lymphoma [15]. This includes multiple myeloma in those who have and have not previously received treatment [16]. It is generally used together with other medications [16]. It is given by injection either intravenously or subcutaneously [15]. Common side effects include nausea, diarrhea, tiredness, thrombocytopaenia, fever, numbness, low white blood cells, and shortness of breath, rash and abdominal pain [15]. Other severe side effects include low blood pressure, tumor lysis syndrome, heart failure, and reversible posterior leukoencephalopathy syndrome [15,16]. It is in the class of medications known as proteasome inhibitor [15]. It works by inhibiting proteasomes [16]. Bortezomib was approved for medical use in the United States in 2003 and in Europe in 2004 [15,16]. It is on the World Health Organization's List of Essential Medicines, the safest and most effective medicines needed in a health system [17].
Epidemiology and cost of Multiple Myeloma treatment at the Cochin Cancer Research Centre
The reported incidence of multiple myeloma in India ranges from 0.5 to 1.2 per 100000 but there have been few studies on the effect of treatment of this condition. We, therefore, analyzed the clinical profile of patients in Kerala with myeloma, the treatment given and the factors affecting survival. This is the most common lymphoreticular neoplasm seen at the Cochin Cancer Research Centre similar to the rest of India [14]. Multiple myeloma is associated with significant lifelong costs to healthcare payers and society. The cost of treatment for the different Bortezomib-based regimens used in Myeloma per cycle according to the National Health Protection Scheme, Ayushman Bharat-Prime Minister Jan ArogyaYojana (AB-PMJAY) also known as Karunya Arogya Suraksha Paddati (KASP) are as follows:
Bortezomib, Lenalidomide and Dexamethasone 20000.00
Bortezomib, Cyclophosphamide and Dexamethasone 10000.00
Bortezomib and Dexamethasone 6000.00
Daratumumab, Ixazomib and Elotuzumab are yet to find its way into the drug repertoire at the Cochin Cancer Research Centre. Drug costs account for most of the treatment costs of multiple myeloma. The cost of treatment in patients eligible for transplant generally appears higher than that in patients ineligible for transplant. The mean annual costs in patients who had lytic bone lesions and underwent radiotherapy or decompression surgery for spinal cord compression was approximately 2.25-fold higher than that in patients who were treated with zoledronic acid along with calcium supplement.
Platelet transfusions
Although studies of the costs to society of multiple myeloma are lacking, multiple myeloma is assumed to be associated with loss-of–productivity costs for patients and their caregivers. Tranfusing 1 unit of packed red cells, 2 units of platelets and 1 unit of Fresh frozen plasma for each thrombocytopaenic episode while on Bortezomib therapy costs 820. The productivity of patients with recurrent thrombocytopaenia would be anticipated to decrease in patients requiring platelet transfusions and is comparatively better with home treatment of mild to moderate thrombocytopaenia with a bypassing agent that effectively and quickly resolves thrombocytopaenia. Moreover, the use of bypassing agents to allow orthopedic surgeries to be performed would be expected to be associated with increases in both productivity and health-related quality of life (HR-QOL).
Clinical profile of Bortezomib
The efficacy and tolerability of intravenous and subcutaneous Bortezomib in the treatment of multiple myeloma patients has been previously reviewed. This section briefly highlights results of studies in multiple myeloma patients receiving clinically relevant dosages of Bortezomib in the on-demand home treatment of mild to moderate thrombocytopaenia either symptomatic or asymptomatic (section 3.1.1) or to maintain haemostasis in Bortezomib induced thrombocytopaenia(section 3.1.2), and its tolerability in these indications (section 3.2). The focus is on studies that were used as a source of input data in pharmacoeconomic analyses in these indications (section 4).
Therapeutic efficacy
Bortezomib is a modified dipeptidyl boronic acid. It is a reversible inhibitor of the 26S, proteasome, a large protein complex that degrades ubiquitinated proteins. Inhibition of the proteasome pathway leads to activation of multiple signaling cascades cell-cycle arrest, and apoptosis. After IV administration, more than 90% of the drug is rapidly cleared from theplas a within minutes and distributed widely to peripheral tissues. The efficacy of first-line therapy in thromboctopaenia controlled with Carica papaya leaf extract was 87.1-100% and 56.7-79% for tranfusing 1 unit of packed red cells, 2 units of platelets and 1 unit of Fresh frozen plasma for each thrombocytopaenic episode while on Bortezomib therapy.
Home treatment of thrombocytopaenia
On-demand treatment with Carica papaya leaf extract at home was effective in controlling mild to moderate thrombocytopaenia [18]. A dramatic increase in the platelet count was seen following the administration of Carica papaya leaf extract. Patients with platelet counts below 80,000 cells/cu mm would experience a rise of up to more than 150,000 / μL in 3 to 4 days’ time. This does not depend on the initial platelet count or the chemotherapy regime administered in almost all the patients with multiple myeloma presenting at the Cochin Cancer Research Centre. Early treatment with Carica papaya leaf extract was associated with a successful outcome. There is a lack of well-designed head-to-head comparative trials of Carica papaya leaf extract and newer agents for correcting thrombocytopaenia like Romiplostim and Eltrombopag in the on-demand treatment of mild to moderate thrombocytopaenia in multiple myeloma patients being treated with Bortezomib-containing regimens.
Maintenance of platelet counts while on Bortezomib therapy
Transfusing random donor platelet concentrates repeatedly for Bortezomib induced thrombocytopaenia may stimulate production of antiplatelet antibodies causing destruction of the transfused platelets [19]. However, most patients prefer oral administration of Carica papaya leaf extract. Patients experience reductions in their platelet count of around 60% and therefore do not usually develop grade 4 thrombocytopenia unless the baseline count is below 70. The thrombocytopenia is transient and reversible showing a cyclical pattern with platelets dipping at day 11 but returning to baseline by day 1 of the next cycle. This is different to the pattern of thrombocytopenia seen with other cytotoxic agents where platelet counts drop after 1-2 weeks and can take up to 4 weeks to recover or may not recover at all. This is due to a difference in the mechanism of thrombocytopenia in proteasome inhibition, which is related to a transient effect on megakaryocyte function and platelet budding as opposed to damage to marrow stem cells [20].
Tolerability
Bortezomib has no effect on stem cells [15] or megakaryocyte maturation but does inhibit nuclear factor kappa B, a critical regulator of platelet shedding [16]. This probably explains the relatively short duration of thrombocytopenia following its administration [16]. Not all chemotherapy drugs reduce platelet production; some can actually increase the rate of platelet destruction. Indeed, platelet survival itself may be altered by some agents.
Pharmacoeconomic analyses of Bortezomib
Cost utility
A review of published studies in 2011 found that, despite advances in therapy for multiple myeloma, the literature at the time was still lacking economic comparisons of novel therapies, specifically cost-effectiveness studies. Since that time, 1 study evaluating the costs of care of multiple myeloma has been published [21]. Durie and colleagues developed an economic model to evaluate the total treatment costs and the monthly costs without progression associated with lenalidomide plus dexamethasone (Rd) versus bortezomib plus dexamethasone [22]. The results of this model demonstrated that the drug and medical costs associated with bortezomib were more than US $17,000 higher than those for patients treated with lenalidomide [22]. At the Cochin Cancer Research Centre, Bortezomib cost varies between 575 per dose to 2000depending on the manufacturer’s retail price. Likewise, Lenalidomide costs between 21 to 75 for a 10mg capsule.
Cost utility in the treatment of transplant-eligible multiple myeloma
Although MM accounts for only a small percentage of all cancer types, the costs associated with treating and managing it are among the highest. Recent developments in diagnosing, treating, and managing myeloma have led to novel treatment strategies. Immunomodulators, proteasome inhibitors, and bisphosphonates are improving response rates and preserving quality of life. However, these agents are not replacing older treatment modalities, but being used in addition to them. Intensive chemotherapy, stem cell transplantation, and supportive care are all important components in achieving treatment goals. Costs associated with stem cell transplants and complications of the disease add to the economic burden of myeloma. Additional costs for routine diagnostics to measure the progression of the disease or response to treatment need to be considered. Complications (e.g., lytic bone disease, infection, anemia, and renal failure) also add to morbidity and mortality, thus increasing the burden to the patient and the health care system as a whole. Financial and time constraints of caregivers must also be considered, as well as the added administrative burdens to health care providers.
Cost utility in the treatment of transplant-ineligible multiple myeloma
The number of published studies that include economic evaluations of transplant-ineligible multiple myeloma has increased in recent years, spurred by the large number of new therapeutic and diagnostic technologies, their associated costs and the limited resources available to pay for them. For the results of clinical and economic evaluations to be used for policy formulation it is important to develop an idea of the orders of magnitude of cost-effectiveness that are likely to be associated with wise adoption and utilization and with unwise use of health care resources. Such a study has not been conducted till now [23].
Cost analyses of the treatment of multiple myeloma
New standards of care in the treatment and management of myeloma are likely to lead to significant increases in costs. Although costs are not the only elements to be considered, they are crucial in the management of this already costly disease. All aspects of myeloma treatment and supportive care must be evaluated and analyzed. Cost of pharmaceuticals alone must not be a driving factor in treatment decisions. Economic analyses can be used to demonstrate that the least expensive alternative is not always the most economical, and that it may not produce an optimal outcome for both the health plan and the patient. Although cost containment is clearly an important objective, quality of care is the first priority, and managed care organizations like the Cochin Cancer Research Centre have the challenge of making balanced cost and benefit assessments.
Methodology
Participants: 1374 chemotherapy schedules with Bortezomib containing regimens in patients on treatment for multiple myeloma. The ethics committee of the Cochin Cancer Research Centre advised that no prior approval is required for analysis of routine data in April 2017.
Materials: A decision model was used which comprised of initial and subsequent treatments for multiple myeloma, the probability of switching from one Bortezomib regime to another, the probability that thrombocytopaenia will resolve with each treatment, probability of recurrent thrombocytopaenic episodes and treatment for thrombocytopaenia or bleeding episodes. A simplified diagram of the general flow of patients in the decision models is presented in figure 1.
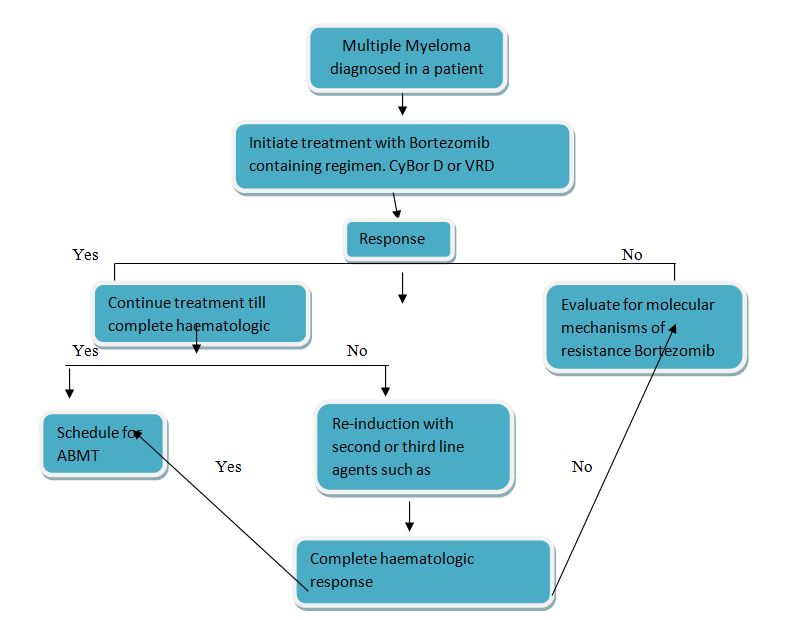
Figure 1: Simplified diagram of patient flow in cost analyses of treatment with Bortezomib and Bortezomib containing regimens in patients with multiple myeloma. ABMT-Autologous Bone Marrow Transplantation.
Design: Descriptive
Method: Use of a checklist of basic criteria for pharmacoeconomic analyses as presented in figure 2.
|
Figure 2: Checklist of basic criteria for pharmacoeconomic analyses as applied to fully published modelled analyses of the costs associated with bortezomib in the treatment of multiple myeloma. |
|||
|
S/N |
Category |
Lifetime analysis in CCRC |
Analysis of the cost of bortezomib per patient in US [24], Turkey [25],, Brazil [26], Russia [27] |
|
1 |
Purpose of study explained? |
|
|
|
2 |
Study perspective mentioned? |
|
|
|
3 |
Comparative treatments relevant/ appropriate? |
|
|
|
4 |
Source of clinical outcomes data stated? |
|
|
|
5 |
Quality of clinical data good? |
a |
a |
|
6 |
Sources of costs and healthcare resource use stated? |
|
|
|
7 |
Unit costs provided |
|
|
|
8 |
Year of costing provided? |
|
|
|
9 |
Discounting applied appropriately? |
|
NA |
|
10 |
Sensitivity analysis conducted |
|
|
|
11 |
Sensitivity analysis performed on key variables and justification given for range of values? |
|
|
|
12 |
Incremental analysis conducted? |
NAb |
NAc |
|
13 |
Discussion of results provided, including study limitations, comparison with previous studies addressing similar issues, etc. |
|
|
|
14 |
Source of funding disclosed? |
|
|
|
15 |
Conclusions valid |
|
|
|
16 |
Overall, is the study clinically relevant? |
|
|
|
a. Although clinical data were derived from a variety of sources and were based on first line treatment of multiple myeloma with bortezomib, the lack of head to head comparative trails and the difficulty in enrolling patients into such studies. b. Conducted on the comparisons between on demand treatment and immune tolerance induction regimes, but not on the comparisons of on-demand regimens, which assumed equivalence efficacy. c. Cost minimization analyses that assumed 100% efficacy after the last stage of treatment CCRC = Cochin Cancer Research Centre NA = Not Applicable |
|||
Results
On demand treatment with Caricapapaya leaf extract for the management of mild to moderate thrombocytopaenia in patients with multiple myeloma was predicted to be associated with lower total medical costs than on-demand treatment with platelet transfusions in patients being treated with Bortezomib containing regimens.
Time?to?event outcomes data
We extracted the hazard ratios (HR) and 95% confidence intervals (CI) for OS and PFS from included studies and calculated the overall odds ratio (OR) and 95% CI for combined studies using methods recommended in the Cochrane Handbook for Systematic Reviews of Interventions [28]. Bortezomib seems to have benefits in terms of overall and progression-free survival, and response rates, but seems to be associated with a number of adverse events [29].
Figure 3: In people with multiple myeloma, how does bortezomib affect outcomes?
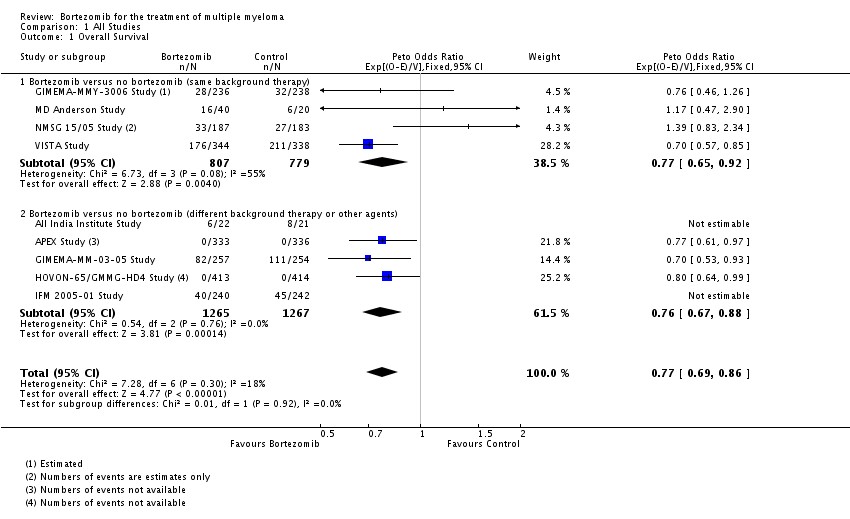
Courtesy: Cochrane library.
Sensitivity analyses
Results predicting that the total treatment costs of Bortezomib associated with platelet transfusions were more than those associated with the use of Carica papaya leaf extract were generally robust to plausible changes in key parameters. Results were usually more sensitive to the efficacy of the treatment modality in resolving thrombocytopaenia, the number of thrombocytopaenic episodes, the number of transfusions needed and the transfusion acquisition cost.
Study strengths and limitations
Pharmacoeconomic analyses of Bortezomib, like all pharmacoeconomic analyses, can be subjected to a number of criticisms. The use of a model in pharmacoeconomic analyses involves the incorporation of costs and clinical outcomes that rely on a number of assumptions. Data obtained from a variety of sources, including clinical trial results that may differ from those obtained in real-life practice, are extrapolated to the general population. Even when results of these analyses are robust to plausible changes in key input variables, results of an anlalysis in one country may not be applicable to other countries because of differences inhealthcare systems, medical practice and unit costs.
Pharmacoeconomic positioning of Bortezomib at the Cochin Cancer Research Centre
Multiple Myeloma is a lifelong disease associated with considerable morbidity and substantial direct and indirect costs (section 2). The number or severity of bleeding episodes does not increase in patients adopting a healthy lifestyle. Although multiple myeloma patients used to have a high mortality rate, including Bortezomib in the treatment of the disease, have contributed to a prolonged life expectancy in this patient population. Treatment with Bortezomib is associated with achievement of a satisfactory HR-QOL in multiple myeloma patients including the transplant-ineligible patients. There were significant reductions in the number of required re-treatments in the transplant-eligible patients, duration of painful episodes, days requiring wheelchair/crutches, emergency-room visits and lost carer time relative to patients’ usual treatment. The orthopaedic status of patients with bone lesions influenced HR-QOL, but other aspects did not appear to have an influence on the global well-being of patients. Pharmacoeconomic analyses that include HR-QOL and productivity data in addition to direct medical cost data would be beneficial in establishing the overall cost effectiveness of Bortezomib in multiple myeloma. The available pharmacoeconomic analyses did not consider the effect of treatment-related adverse effects on costs, but this is unlikely to have a marked effect on the overall cost effectiveness of Bortezomib relative to Lenalidomide.
Implications for research
While substantial clinical evidence has accumulated to support the use of bortezomib as a treatment for multiple myeloma, clinical trials of newer proteasome inhibitors are also needed. A number of novel proteasome inhibitor drugs are in clinical development, the most advanced of which is carfilzomib. A global assessment of novel agents should encompass not only survival and response outcomes but also adverse effects and patient quality of life. In addition, given the increasing cost of anti-cancer therapies on Indian health budgets, a formal evaluation of the cost-effectiveness of these newer proteasome inhibitor drugs should be routinely included in cost-benefit analyses. In summary, further research should encompass the following.
- The optimal proteasome inhibitor to be included in combination regimens for the treatment of myeloma in each disease and therapy setting.
- Further evaluation of clinical and biologic prognostic markers, for example fluorescence in situ hybridisation (FISH) cytogenetic profiles and their influence on response to treatment with proteasome inhibitors.
- Further evaluation on the dose and scheduling of proteasome inhibitors in order to improve the toxicity profile of this class of agent and for optimal health-related quality of life.
- Mechanisms of resistance to proteasome inhibitors should be identified and strategies to overcome resistance developed.
- Predictors of response to proteasome inhibitor treatment should be identified, such that treatment can be tailored to individual myeloma patients.
References
1. Raab MS, Podar K, Breitkreutz I, et al. Anderson KC (July 2009).Multiple myeloma. Lancet. 374: 324-329.
2. Plasma Cell Neoplasms (Including Multiple Myeloma). Patient Version. NCI. 1980-01-01
3. Plasma Cell Neoplasms (Including Multiple Myeloma) Treatment (PDQ®) Health Professional Version. NCI. 2016. 4. World Cancer Report 2014. World Health Organization. 2014. 5. World Cancer Report 2014. World Health Organization. 2014.
6. Plasma Cell Neoplasms (Including Multiple Myeloma) Treatment. National Cancer Institute. 1980.
7. Ferri, Fred F. 2013. Ferri's Clinical Advisor 2014 E-Book: 5 Books in 1. Elsevier Health Sciences. 726.
8. van deDonk NW, Mutis T, Poddighe PJ, et al. 2016. Diagnosis, risk stratification and management of monoclonal gammopathy of undetermined significance and smoldering multiple myeloma. International Journal of Laboratory Hematology. 38 Suppl 1: 110-122. Ref.: https://bit.ly/3bIM9Y4
9. GBD 2015 Disease and Injury Incidence and Prevalence, Collaborators. (8 October 2016). Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 388: 1545-1602.
10. GBD 2015 Mortality and Causes of Death, Collaborators. (8 October 2016). Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 388: 1459-1544.
11. SEER Stat Fact Sheets: Myeloma. NCI Surveillance, Epidemiology, and End Results Program. Archived from the original on 27 July 2016.
12. Diepenbrock, Nancy H. 2011. Quick Reference to Critical Care. Lippincott Williams & Wilkins. 292.
13. Natl Med J India 1993. 6: 7-10
14. Bortezomib Monograph for Professionals. Drugs.com
15. Velcade. European Medicines Agency. 17 September 2018.
16. World Health Organization. 2019. World Health Organization model list of essential medicines: 21st list 2019. Ref.: https://bit.ly/3bGl87K
17. Prem Ravi Varma PK. 2019. Thrombopoiesis in ChemotherapyInducedThrombocytopaenia Using Carica Papaya Leaf Extract -An Attentive Clinical Observation. BAOJ Cancer Res Ther 5: 68.
18. Catalano PM. 1992. NMS Hematology. Malvern, Pennsylvania. Harwal Publishing Company.
19. Messori A, Maratea D, Nozzoli C, et al. 2011. The role of bortezomib, thalidomide and lenalidomide in the management of multiple myeloma: an overview of clincial and economic information.Pharmacoeconomics. 29: 269-285. Ref.: https://bit.ly/2R5IfRo
20. Lonial S, Waller EK, Richardson PG, et al. 2005. Risk factors and kinetics of thrombocytopenia associated with bortezomib for relapsed, refractory multiple myeloma.Blood. 106: 3777-3784. Ref.: https://bit.ly/343Q3Ze
21. Durie B, Binder G, Pashos C, et al. 2013. Total cost comparison in relapsed/refractory multiple myeloma. J Med Econ. 16: 614-622. Ref.: https://bit.ly/39BMgmM
22. Cost effectiveness and strategic planning (WHO-CHOICE). 23. Scott K, Hayden PJ, Will A. 2016. Bortezomib for the treatment of multiple myeloma. Cochrane Systematic Review - Intervention Version published: 20. Ref.: https://bit.ly/3azEZFD
24. Roy A, Kish JK, Bloudek L, et al.2015. Estimating the Costs of Therapy in Patients with Relapsed and/or Refractory Multiple Myeloma: A Model Framework. Am Health Drug Benefits. 8: 204-215. Ref.: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4489189/
25. Ludwig H, Beksac M, Bladé J, et al. 2011. Multiple myeloma treatment strategies with novel agents in 2011: a European perspective. Oncologist. 16: 388-403. Ref.: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3228121/
26. Clark L, Castro AP, Fortes AF, et al. 2011. Ideal vial size for bortezomib: real-world data on waste and cost reduction in treatment of multiple myeloma in Brazil. Value Health. Jul-Aug. 14: 82-84. Ref.: https://bit.ly/2UUZBBo
27. Avksentieva, M et al. PHM3 clinicoeconomical analysis of Bortezomib vs dexamethasone in recurrent or treatment-resistant multiple myeloma in Russia.Value in Health. 10. Ref.: https://bit.ly/3bMBoEl
28. Higgins JPT, Altman DG, Sterne JAC. 2011. Chapter 16: Special topics in statistics. Cochrane Handbook for Systematic Reviews of Interventions. 5. Available from http://www.cochrane?handbook.org: The Cochrane Collaboration.
29. Jane Burch, SimonaNistor?Grahl. In people with multiple myeloma, how does bortezomib affect outcomes? Cochrane clinical answers.16 November 2016.




















