Indexing & Abstracting
Full Text
Research ArticleDOI Number : 10.36811/ijia.2020.110002Article Views : 13Article Downloads : 14
Comparison and Detection of biofilms layers in Staphylococcus aureus and Escherichia coli Isolated from Clinical Specimens
Saptarshi Pal
Department of Microbiology and Virology (Head - Professor E.S. Gorovitz), Russia
*Corresponding Author: Saptarshi Pal, Scientific advisor- Associate Professor Anatoliy Petrovich Godovalov, Department of Microbiology and Virology (Head - Professor E.S. Gorovitz) «Acad. E.A. Wagner Perm State Medical University», Perm, Russia, Email: bpalsas@gmail.com
Article Information
Aritcle Type: Research Article
Citation: Saptarshi Pal. 2020. Comparison and Detection of biofilms layers in Staphylococcus aureus and Escherichia coli Isolated from Clinical Specimens. Int J Immunol Allergy. 1: 09-12.
Copyright: This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Copyright © 2020; Saptarshi Pal
Publication history:
Received date: 20 January, 2020Accepted date: 17 February, 2020
Published date: 19 February, 2020
Abstract
Biofilms are the community that probably represents the mode of growth for microbes in most environments. Biofilms are typically surrounded by an extracellular matrix that provides structure and protection to the community. The mechanisms that different bacteria employ to form biofilms vary, frequently depending on environmental conditions and specific strain attributes. Biofilm formation begins with attachment of bacteria to biotic surface such as host cell. After attachment, aggregation of bacteria is started by cell-cell adhesion. Dispersion is started by certain conditions such as phenol-soluble modulinos. For studying and comparing the different layers of biofilm formation in our model organism like Staphylococcus and Escherichia we used slide detection technique and the extent of biofilm formation is measured using the crystal violet (CV) dye. Using these bacteria as examples, we compared the key sizes of 3 layers of the biofilms as well as effect of antibiotics on these layers.
Keywords: Biofilm; Staphylococcus aureus; Escherichia coli
Introduction
Biofilm is a microbial community which is embedded in extracellular matrix channel. They are unaffected by the defense mechanisms of phagocytosis and antibiotic treatment. The ability of a microorganism to develop biofilm is due to virulence factor which cause many chronic infections. They have emerged as multidrug resistant strains resulting in treatment failure. For better detection especially chronic infections, nowadays it is necessary to detect production of biofilm. Microtiter plate method, tube adherence method and Congo red stain method, are various methods of detection. Both gram positive and gram-negative bacteria have the ability to form biofilms. Bacteria which are commonly involved include E. faecalis, S. aureus, S. epidermidis, E. coli, K. pneumoniae, and P. aeruginosa. Our present study was undertaken with the aim to detect the structure of gram-positive and gram-negative bacterial biofilms by slide detection method. The size of layers of biofilm can range from single cell layer to substantial layer which is encased in polymeric environment. Staphylococcus aureus can produce a multilayered biofilm embedded with a glycocalyx with heterogeneous protein expression while Escherichia coli can produce a constantly hydrated viscous layer protecting embedded bacteria host defenses by preventing recognition of biofilm bacteria by immune system. Antibiotic treatment can change the size of layers and other physical factors or can eliminate the complete biofilm population. Several antibiotics like oxacillin, vancomycin have reduced penetrations throughout layers of Sauers and E. coli.
Materials and Methods
For growing a biofilm firstly, we brought two slides and gently heated it over the Bunsen burner. We had grown a culture of the Staphylococcus aureus and Escherichia coli in a rich medium for 48 hours at 37°C. Meanwhile we gently submerged the slides in a small tub of water for washing. Then we used Gram method for staining biofilms. Then at the last step we observed it under the microscope (x100). We measured the layers in Scope Photo 3 times every layer and calculated its mean and error.
Results
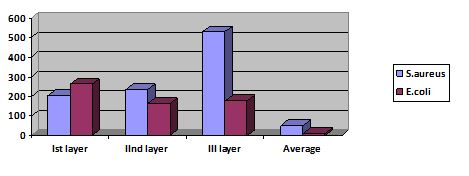
After performing the above steps, we have obtained the following results. Biofilms consist of several layers and contain channels and chambers. This composition depends on strains. We established 4 layers in which the names of the layers were: Ist layer- Layer of new paricles, IInd layer- Layer of channels, IIIrd layer- Dark layer (matrix production), IV th layer- Layer of per sisters. Average size of Ist layer of S. aureus biofilm- 203,30±47,01 μm and for E. coli - 261,51± 54,09 μm (p>0,05). Average size of IInd layer of S. aureus biofilm - 235,37 ± 93.49 μm and for E. coli - 163,85±87,97 μm (p>0,05). Average size of IIIrd layer of S. aureus biofilm - 531, 39±61,04 μm and for E. coli - 177,94 ± 31,96 μm (p<0,05). Average size of channel of S. aureus biofilm - 51,80±35,00 μm and for E. coli - 12,30±8,02 μm (p>0,05).
Discussion
The first biofilm was discovered by Antony Von Leeuwenhoek in 17th century when he saw his microbial aggregates of his plaques of teeth. Then the term “Biofilm” was coined by Bill Costerton in 1978. There are various methods to detect biofilm production like Tissue Culture Plate (TCP), Tube method (TM), Congo Red Agar method (CRA), bioluminescent assay, piezoelectric sensors, and fluorescent microscopic examination. By our opinion S. aureus needs more matrix production than E. coli and use this mechanism as protection from antibiotic action. S. aureus can produce a multilayered biofilm embedded within glycocalyx and so it is antimicrobial resistant. The ability of Staphylococcus aureus to produce a biofilm is an important factor for long term persistence bacteria and also can decrease the efficacy of antibiotic therapy. It can also be identified by the presence of genes participating in the biofilm activity. Sauers has a largest IIIrd layer while the E. coli has largest Ist layer when we compared the layers of the biofilms [1-23].
Conclusions
From these results, it can be concluded that Ist and IInd layer of both the biofilms are of same size but the 3rd layer is about 5 times bigger in the S. aureas as compared to E. coli. Biofilm contains a layer but different microorganisms have same structure of layer and we have found that the size of S. aureus have bigger third layer than E. coli.
References
1. Dall’ Antonia M, Wilks M. 2005. between methicillin sensitive and resistant Staphylococcus aureus. 61: 62-67. Ref.: https://bit.ly/2HoLEp1
2. Reba Kanungo. Ananthanarayan and Paniker textbook of Microbiology (10th edition). 2017. Hyderabad: Universities Press Pvt. Limited. 201-208,280-282. Ref.: https://bit.ly/37xN7nr
3. Guilar B, Iturralde M. 2001. Binding of a surface protein of Staphylococcus aureus to cultured ovine mammary gland epithelial cells. Vet Microbiol. 82: 165-175. Ref.: https://bit.ly/2Sra8nZ
4. Cramton SE, Ulrich M, Gotz F, et al. 2001. Anaerobic conditions induce expression of polysaccharide intercellular adhesin in Staphylococcus aureus and Staphylococcus epidermidis. Infect Immun. 69: 4079-4085. Ref.: https://bit.ly/3bF41nx
5. Donlan RM, Costerton JW. 2002. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev.15: 167-193. Re.: https://bit.ly/38v2r5Q
6. Holden MT, Feil EJ, Lindsay JA, et al. 2004. Complete genomes of two clinical Staphylococcus aureus strains: evidence for the rapid evolution of virulence and drug resistance. Proc Natl Acad Sci USA. 101: 9786-9791. Ref.: https://bit.ly/3bDIR9f
7. Joyce JG, Abeygunawardana C, Xu Q, et al. 2003. Isolation, structural characterization and immunological evaluation of a high-molecular-weight exopolysaccharide from Staphylococcus aureus. Carbohydr Res. 338: 903-922. Ref.: https://bit.ly/38uTglJ
8. Karen Smith, Ana Perez, Gordon Ramage, et al. 2008. Biofilm formation by Scottish clinical isolates of Staphylococcus aureus. Journal of Medical Microbiology. 57: 1018-1023. Ref.: https://bit.ly/37v0ULN
9. Indrawattana N, Sungkhachat O, Sookrung N, et al. 2013. Staphylococcus aureus Clinical Isolates: Antibiotic Susceptibility, Molecular Characteristics, and Ability to Form Biofilm. Bio Med Research International. 1-11. Ref.: https://bit.ly/38wciIt
10. Michael Otto. 2008. Staphylococcal Biofilms. Curr Top Microbiol Immunol. 322: 207-228. Ref.: https://bit.ly/3bILEOO
11. Chinithung Ngullie, Ashma Khatun, Varsha A. et al. 2017. Detection of Biofilm Formation in Multi-Drug Resistant Staphylococcus aureus from Rural Tertiary Care Hospital in North IndiaInt. J. Curr. Microbiol. App. Sci. 6: 522-526. Ref.: https://bit.ly/2uDe5go
12. Jamal M, Ahmad W, Andleeb S, et al. 2018. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 81: 7-11. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/29042186
13. Giannelli M, Landini G, Materassi F, et al. 2017. Effects of photodynamic laser and violet-blue led irradiation on Staphylococcus aureus biofilm and Escherichia coli lipopolysaccharide attached to moderately rough titanium surface: in vitro study. Lasers Med. Sci. 32: 857-864. Ref.: https://bit.ly/3bEvK7M
14. Vollmerhausen TL, Conneely A, Bennett C, et al. 2017. Visible and UVA light as a potential means of preventing Escherichia coli biofilm formation in urine and on materials used in urethral catheters. J. Photochem. Photobiol.170: 295-303. Ref.: https://bit.ly/2HqovCG
15. Baselga R, Albizu I, De La Cruz M, et al. 1993. Phase variation of slime production in Staphylococcus aureus: implications in colonization and virulence. Infect Immun. 61: 4857-4862. Ref.: https://bit.ly/3bGrtAM
16. National Institutes of Health. 1999. SBIR/STTR Study and control of microbial biofilms (PA-99-084). Published April 21.
17. Livornese LL, Korzeniowski OM. 1992. Pathogenesis of infective endocarditis. In: Kaye, D, ed. Infective Endocarditis. 2nd ed. New York, NY: Raven Press. 19-35.
18. Bendouah Z, Barbeau J, Hamad WA, et al. 2006. Use of an in vitro assay for determination of biofilm-forming capacity of bacteria in chronic rhinosinusitis. Am J Rhinol. 20: 434-438. Ref.: https://bit.ly/2vDjuUs
19. Muenks CE, Hogan PG, Wang JW, et al. 2016. Diversity of Staphylococcus aureus strains colonizing various niches of the human body. J Inf Secur. 72: 698-705. Ref.: https://bit.ly/31Yp2Fb
20. Tuchscherr L, Heitmann V, Hussain M, et al. 2010. Staphylococcus aureus small-colony variants are adapted phenotypes for intracellular persistence. J Infect Dis. 202: 1031-1040. Ref.: https://bit.ly/2UTnOd9
21. Molina JM, Cordoba J, Ramirez P, et al. 2008. Automatic detection of bacterial and fungal infections in blood. Enferm Infecc Microbiol Clin. 26: 75-80. Ref.: https://bit.ly/2UVjxFY
22. Pilecky M, Schildberger A, Orth-Höller D, et al. 2019. Pathogen enrichment from human whole blood for the diagnosis of bloodstream infection: prospects and limitations. Diagn Microbiol Infect Dis. 94: 7-14. Ref.: https://bit.ly/2tYdrcM
23. Sarkar P, Yarlagadda V, Ghosh C, et al. 2017. A review on cell wall synthesis inhibitors with an emphasis on glycopeptide antibiotics. Medchemcomm. 8: 516-533. Ref.: https://rsc.li/2StXMeM




















