Indexing & Abstracting
Full Text
Research ArticleDOI Number : 10.36811/irjo.2020.110006Article Views : 2Article Downloads : 3
Evaluation of Intravital Ranibizumab on Proliferative Diabetic Retinopathy with Macular Edema by Optical Coherence Tomography Angiography
Mona Abdelkader1,*, Hamza Abdelhamed1, Ebtihal Abdelaziz2 and Amr Abdelkader3
1Professor of Ophthalmology, Faculty of Medicine - Mansoura University, Egypt
2Resident in Mansoura ophthalmic center, Faculty of medicine, Mansoura University, Egypt
3Lecturer of Ophthalmology, Faculty of Medicine- Mansoura University, Egypt
*Corresponding Author: Mona Abdelkader, MD, Ophthalmology Center, Faculty of Medicine, Mansoura University, Mansoura, Egypt, 35516, Tel: 002-050-2202064; 00201006278757; Fax 002-050-2256104; Email: monaabdelkader78@yahoo.com
Article Information
Aritcle Type: Research Article
Citation: Mona Abdelkader, Hamza Abdelhamed, Ebtihal Abdelaziz, et al. 2020. Evaluation of Intravital Ranibizumab on Proliferative Diabetic Retinopathy with Macular Edema by Optical Coherence Tomography Angiography. Int Res J Optha. 2: 14-25.
Copyright: This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Copyright © 2020; Mona Abdelkader
Publication history:
Received date: 09 November, 2020Accepted date: 17 November, 2020
Published date: 19 November, 2020
Abstract
Purpose: To evaluate the effect of ranibizumab by Optical Coherence Tomography Angiography (OCTA)in proliferative diabetic retinopathy (PDR) with macular edema and to evaluate the role of Swept Source Optical Coherence Tomography Angiography in detection and characterization of new vessels at the disc (NVD) and new vessels elsewhere (NVE).
Subjects and Method: The study included patients with proliferative diabetic retinopathy (PDR) and clinically significant diabetic macular edema. Patients with central macular thickness (CMT) above 300 μm underwent intravitreal injection of 0.5 mg Ranibizumab& those having CMT below 300μm had laser treatment.
Results: The study was conducted on 50 cases with proliferative diabetic retinopathy with macular edema. There was significant increase in best corrected visual acuity (BCVA) across time of treatment. Mean (±SD) BCVA at diagnosis, at 1 month and at 3 months were 9.1±0.1, 0.8±0.1, 0.7±0.2. respectively (p<0.001). Central macular thickness (CMT); Area of neovascularization (ArNV); Exuberant Vascular Proliferation (EVP)showed significant decrease across time of treatment.
Conclusion: ranibizumab injections are a safe and effective alternative to Pan retinal photocoagulation for the management of PDR. OCTA is useful to monitor different NVD subtypes, their development, the efficacy of treatment regimens as well as to define new vessel morphological details. Small scan size delineates minute morphology of NVD and misses extension of lesions beyond disc margin, while larger scan size masks fine details.
Introduction
Proliferative diabetic retinopathy PDR is considered one of the main causes of vision loss in patients with diabetes mellitus [1]. Optical coherence tomography angiography (OCTA) is a novel non-invasive imaging modality for 3-dimensional visualization of retinal and optic nerve capillary networks without the injection of exogenous dyes as compared with fluoresceine angiography (FA) [2]. OCT angiography can visualize the micro-vascular structures of neovascularization and can also directly visualize well-delineated micro vascular structures of new vessels and differentiate them from original retinal vascular structures [3]. Optical coherence tomographic angiography (OCTA) uses motion contrast imaging and works by comparing OCT B-scans acquired repeatedly at a given retinal location [4]. Injection of anti-VEGF drugs could lead to a pro -fibrotic switch with significant reduction in the neo -vascular component [5]. Panretinal photocoagulation (PRP) is the standard treatment for PDR since the Diabetic Retinopathy Study have shown its efficacy nearly 40 years ago as it reduces vascular endothelial growth factor(VEGF) [6]. However, PRP has its drawbacks such as permanent peripheral visual field loss, decreased night vision and also worsening of diabetic macular edema which trigger researchers for using intra vitreous anti-VEGF agents [7]. Intravitreal injection of anti-vascular endothelial growth factor (VEGF) agents have become an interesting alternative for new vessels regression without photocoagulation of peripheral retina. Anti-VEGF reduce the risk of diabetic retinopathy worsening and increase the chance of improvement [8]. Ranibizumab is a high affinity recombinant Fab, which neutralizes all isoforms of VEGF [9].
Subjects and Methods
Patients with proliferative diabetic changes with clinically significant diabetic macular edema were included in the study. Patients with central macular thickness (CMT) above 300 μm underwent IVI of 0.5 mg Ranibizumab& those having CMT below 300μm had laser treatment. The study protocol was approved by medical research ethics committee, faculty of medicine, Mansoura University. Informed consent was obtained from each participant in the study after assuring confidentiality.
Exclusion criteria
Macular scar, macular hole, uveitis, neo-vascular glaucoma, age related macular degeneration, patients that had undergone treatment for diabetic macular edema (vitrectomy), previous laser treatment, previous intravitreal anti-VEGF or steroid, patients that have relevant malignant systemic disease associated with increase in systemic VEGF and Media opacity that does not permit optical coherence tomography angiography acquisition with good signal strength are excluded from study. Patients underwent complete ophthalmological examination and optical coherence tomography angiography (OCT).
Optical Coherence Tomography Angiography (OCT)
Imaging was performed with SS-OCT at 100 000 A-scans per second (DRI triton OCT, Topcon, Tokyo, Japan). Mydriatic eye drops were used to assure maximal OCT signal and analysis in patients eyes prior to OCT examination. The patient was asked to fixate on a target point inside the instrument. The phase is completed by a camera, located inside the instrument that displays the fundus and scan beam. After the patient scanning was finished, analysis protocol was used to obtain circular maps on the fovea. Green means thickness within normal according to sex and age, yellow means moderate thinning, red means sever thinning, orange means moderate thickening, and purple means sever thickening. The resulting OCT angiogram were evaluated for NVD in vitreous scans, superficial scans of optic nerve vasculature and radial peri papillary capillaries. Also, composite images of blood flow overlay on cross sectional OCT and blood vessel density maps were evaluated. As regarding NVE, enface images of superficial capillary plexus and deep capillary plexus were examined.
Treatment with pan retinal photo coagulation
According to DRS protocol using a standard argon-type laser PRP, settings include burns that range approximately 200μ to 500μ in size, durations of 100 milliseconds, and 200-250 mW of power behind each fire of the laser. The goal is to produce burns that are grey in color; avoid white burns. the edges of the burns are distributed about 1 burn width apart. The distribution of burns should avoid the optic disk (to reduce the risk of thermal damage to the optic nerve) and the macula (to reduce the risk of impairing central vision). Laser burns are generally not placed within the temporal retinal vessel arcades, and the burns extend anteriorly at least to the equator of the retina. Treatment with pan retinal photocoagulation can be completed in one or more settings, depending on the patient’s tolerance for the discomfort of the laser. If more than one sitting is required, the course of therapy is usually completed within a month after the initiation of treatment. follow-up evaluation usually occurs within the first month and then 3 months after the completion of treatment in order to confirm that the neovascularization either regresses or stabilizes.
Intravitreal injection of Ranibizumab
Using aseptic technique, all of the Lucentis vial contents are withdrawn through a 5-micron (19-gaugex 1-1/2 inch), sterile filter needle attached to a 1 mL syringe (not included). Then the filter needle discarded after withdrawal of the vial contents and not to be used for intra vitreal injection. The filter needle was then replaced with a sterile 30-gauge x ½ inch needle for the intra vitreal injection. The data for BCVA, CMT, proliferative changes and the adverse effects were analyzed before, 1 month and 3 months after PRP or intravitreal injection with Ranibizumab.
Statistical analysis
The collected data was revised, coded, tabulated and introduced to a PC using Statistical package for Social Science (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp.). Shapiro test was done to test the normality of data distribution. Paired sample t test was used to assess changes in parameters over time. Correlation analysis to assess the strength of association between two quantitative variables.
Results
The study was conducted on 50 cases with proliferative diabetic retinopathy with macular edema. Age, gender were included in table 1. Mean central macular thickness (CMT) was 331.5±69. Exuberant Vascular Proliferation (EVP) was present in all studied cases (100%). NVD was detected in 26 cases (52%) and NVE in 14 cases (28%). Both NVD and NVE were detected in in 10 cases (20%). PRP was applied in 29 cases (58%) &intra-vitreal injection (IVI) of Ranibizumab was applied in 21 cases (42%). Higher CMT was significantly associated with NVE. Otherwise, no significant associations were found in studied parameters according to proliferative diabetic changes shown by OCTA in all cases as in table 2.
There was significant increase in BCVA (using LogMAR way) across time of treatment, mean (±SD) BCVA at diagnosis was 0.91±0.1. At 1 month of treatment, it was 0.8±0.1, and at 3 months after treatment, it was 0.7±0.2. (p<0.001).
CMT showed significant decrease across time of treatment, mean (±SD) CMT at diagnosis was 331.5±69. At 1 month of treatment, it was 287.2±55.1, and at 3 months after treatment, it was 241.9±39.4. (p<0.001).
|
Table 1: Demographic features in all studied cases. |
||
|
|
Number of Eyes= 50 |
|
|
Mean |
SD |
|
|
Age (years) |
53.1 |
10.1 |
|
|
Number |
% |
|
Males |
29 |
58 |
|
Females |
21 |
42 |
|
Left eye |
27 |
54 |
|
Right eye |
23 |
46 |
|
IOP (mm Hg) |
13.8 |
1.8 |
|
BCVA |
0.9 |
0.1 |
|
CMT |
331.5 |
69 |
|
ArNV |
1.1 |
0.2 |
|
Present EVP |
50 |
100% |
|
NVD only |
26 |
52% |
|
NVE only |
14 |
28% |
|
Both NVD and NVE |
10 |
20% |
|
PRP |
29 |
58 |
|
IVI of ranbizumab |
21 |
42 |
|
IOP, Intra ocular pressure; BCVA, Best Corrected Visual Acuity; CMT, Central Macular Thickness; ArNV, Area of neovascuarizaton; EVP, Exuberant Vascular Proliferation; NVD, new vessels at the disc; NVE, new vessels elsewhere. |
||
|
Table 2: Comparison of proliferative diabetic changes (NVD and NVE) by OCTA in studied cases. |
|||||||
|
NVD N=26 |
NVE N=14 |
Both NVD and NVE N =10 |
P |
||||
|
Age (years) |
54.0 |
8.0 |
52.1 |
11.3 |
52.1 |
13.7 |
0.813 |
|
Males |
14 |
53.8% |
8 |
57.1% |
7 |
70.0% |
0.692 |
|
Females |
12 |
46.2% |
6 |
42.9% |
3 |
30.0% |
|
|
Left eye |
12 |
46.2% |
9 |
64.3% |
6 |
60.0% |
0.565 |
|
Right eye |
14 |
53.8% |
5 |
35.7% |
4 |
40.0% |
|
|
DM duration (years) |
10.9 |
3.3 |
9.4 |
3.1 |
9.0 |
2.2 |
0.453 |
|
Hypertension duration (years) |
7.2 |
2.6 |
6.4 |
2.6 |
8.0 |
2.6 |
0.818 |
|
HbA1C (%) |
9.1 |
0.8 |
9.0 |
0.5 |
9.4 |
0.7 |
0.519 |
|
IOP (mm Hg) |
13.5 |
1.5 |
14.3 |
1.9 |
14.0 |
2.3 |
0.409 |
|
BCVA |
0.9 |
0.1 |
0.9 |
0.1 |
0.8 |
0.1 |
0.188 |
|
CMT |
301.9 |
56.4 |
369.4 |
76.0 |
355.5 |
58.1 |
0.004 |
|
ArNV |
1.1 |
0.2 |
1.0 |
0.2 |
1.2 |
0.2 |
0.735 |
|
Present EVP |
26 |
100.0% |
14 |
100.0% |
10 |
100.0% |
- |
|
PRP |
18 |
69.2% |
7 |
50.0% |
4 |
40.0% |
0.223 |
|
IVI of ranbizumab |
8 |
30.8% |
7 |
50.0% |
6 |
60.0% |
|
|
Table 3: Comparison of BCVA, CMT and ArNV across time in studied cases. |
|||||||
|
|
Before treatment |
1m after treatment |
3m after treatment |
P1 |
P2 |
P3 |
|
|
BCVA |
mean±SD |
0.9±0.1 |
0.8±0.1 |
0.7±0.2 |
<0.001 |
<0.001 |
<0.001 |
|
CMT |
mean± SD |
331.5±69 |
287.2±55.1 |
241.9±39.4 |
<0.001 |
<0.001 |
<0.001 |
|
ArNV |
mean± SD |
1.1±0.2 |
0.6±0.2 |
0.3±0.1 |
<0.001 |
<0.001 |
<0.001 |
|
EVP |
|
Present |
decreased |
Decreased |
|
|
|
|
N (%) |
50 (100%) |
50 (100%) |
50 (100%) |
<0.001 |
<0.001 |
<0.001 |
|
|
P1 significance at time of treatment, P2 significance at one month of treatment, P3 significance after3months of treatment. |
|||||||
ArNVD showed significant decrease across time of treatment, mean (±SD) ArNVD at diagnosis was 1.1±0.2. At 1 month of treatment, it was 0.6±0.2, and at 3 months after treatment, it was 0.3±0.1. (p<0.001).
EVP showed significant decrease across time of treatment (p<0.001) as shown in table 3. In those treated with PRP, BCVA showed significant increase across time of treatment, (p<0.001). CMT showed significant decrease across time of treatment. ArNV and EVP showed significant decrease across time of treatment. table4, figure 1.
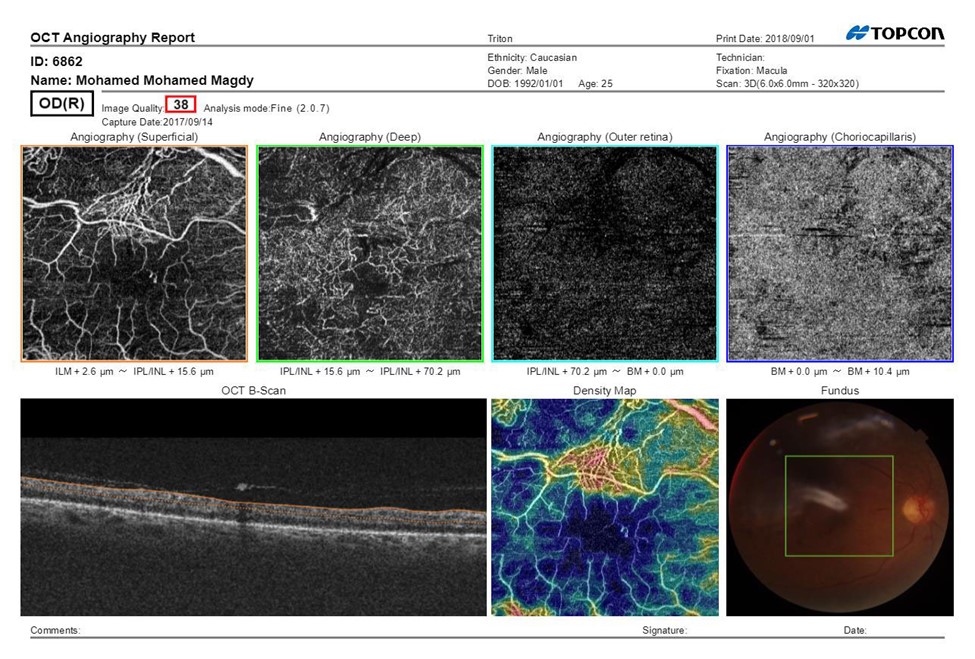
Figure 1A: SS-OCTA scan print out of the macula pretreatment of Male patient aged 45 years having NIDDM since 5 years, BCVA (Log MAR):0.8 HbA1C: 8.7, IOP:16.5 mmHg, CMT pretreatment: 290 μm received PRP, fundus examination shows NVE, Mean area of NVE (μm) pretreatment: 0.98.
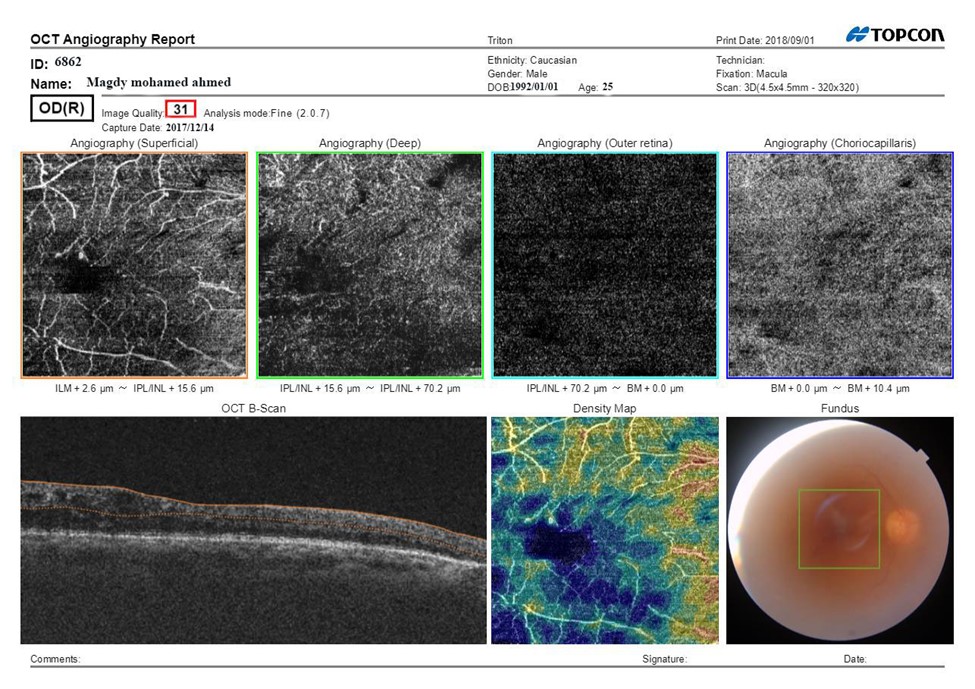
Figure 1B: SS-OCTA scan print out of the macula after treatment after treatment BCVA: 0.6, CMT: 198 μm.
|
Table 4: Comparison of BCVA, CMT and ArNV across time in cases treated with PRP. |
|||||||
|
|
Before treatment |
1m after treatment |
3m after treatment |
P1 |
P2 |
P3 |
|
|
BCVA |
mean±SD |
0.9±0.1 |
0.75±0.1 |
0.67±0.1 |
0.001 |
0.001 |
<0.001 |
|
CMT |
mean±SD |
310.3±56.4 |
272.9±42.2 |
232.5±33.8 |
0.001 |
0.001 |
<0.001 |
|
ArNV |
mean±SD |
1.1±0.2 |
0.5±0.1 |
0.3±0.1 |
0.001 |
0.001 |
<0.001 |
|
EVP |
|
Present |
decreased |
decreased |
|
|
|
|
N (%) |
29 (100%) |
29 (100%) |
29 (100%) |
0.001 |
0.001 |
<0.001 |
|
|
P1 significance at time of treatment, P2 significance at one month of treatment, P3 significance after3months of treatment. |
|||||||
The superficial vascular plexus in the macula showed ischemic areas, widening and irregularities in the fovea avascular zone (FAZ) and tufts of neo vascular proliferation with presence of EVP which can be identified as irregular proliferation of small-caliber new vessels before treatment which turned into pruned vascular loops of filamentous new vessels but does not have lesions of EVP .The deep vascular plexus showed capillary drop out in the deep capillary network of the inner retina. The outer retina layer was free of blood vessels in the macula. The choriocapillaris showed capillary drop out. The B-scan of the macula showed thickening with CMT 331.5μm pretreatment that decreased to 241.9μm at the end of treatment .The vessel density map showed decreased vessel density. The fundus image showed the location of the scan in the macula, hemorrhage, exudate and NVE in those treated with IVI of ranibizumab. BCVA showed significant increase across time of treatment, (p<0.001). CMT, ArNV and EVP showed significant decrease across time of treatment. (p<0.001) table5, figure 2.
|
Table 5: Comparison of BCVA, CMT and ArNV across time in cases treated with IVI of Ranbizumab. |
|||||||
|
|
Before treatment |
1m after treatment |
3m after treatment |
P1 |
P2 |
P3 |
|
|
BCVA |
mean±SD |
0.8±0.1 |
0.65±0.1 |
0.59±0.2 |
<0.001 |
<0.001 |
<0.001 |
|
CMT |
mean±SD |
360.7±75.3 |
306.9±65.2 |
254.9±43.5 |
<0.001 |
<0.001 |
<0.001 |
|
ArNV |
mean±SD |
1.2±0.2 |
0.6±0.1 |
0.4±0.1 |
<0.001 |
<0.001 |
<0.001 |
|
EVP |
|
Present |
Decreased |
Decreased |
|
|
|
|
N (%) |
21 (100%) |
21 (100%) |
21 (100%) |
<0.001 |
<0.001 |
<0.001 |
|
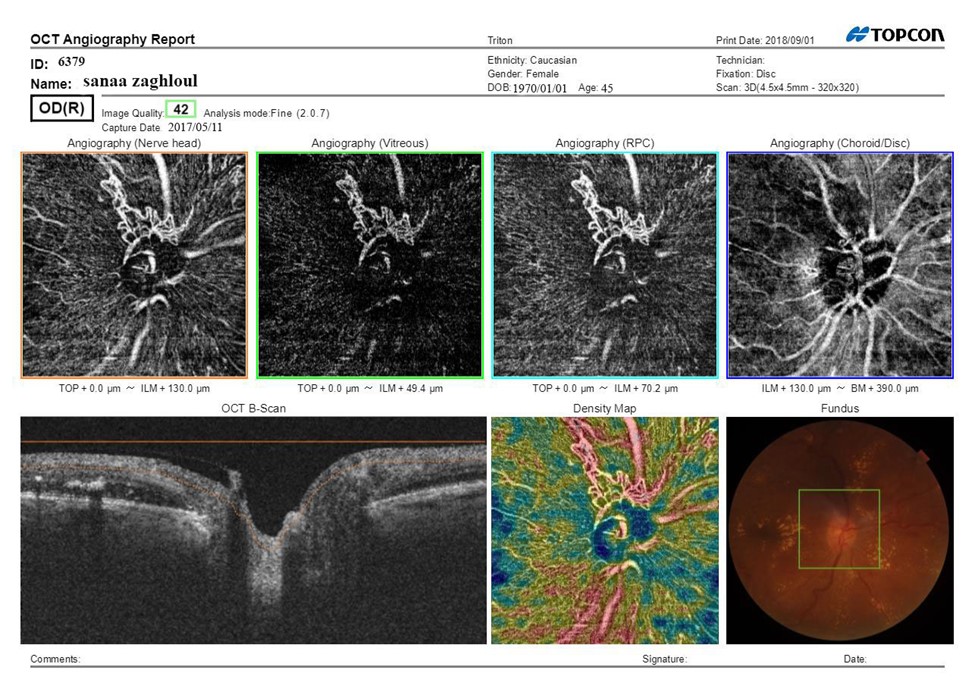
Figure 2A: SS-OCTA scan print out of optic disc pretreatment of female patient aged 45 years having IDDM since 5 years, hypertensive since 5 years, BCVA (Log MAR):0.8 HbA1C: 10.7, IOP:16.5 mmHg, CMT pretreatment: 420 μm received IVI of 0.5mg of Ranibizumab fundus examination shows NVD, Mean area of NVD (μm) pretreatment:1.1.
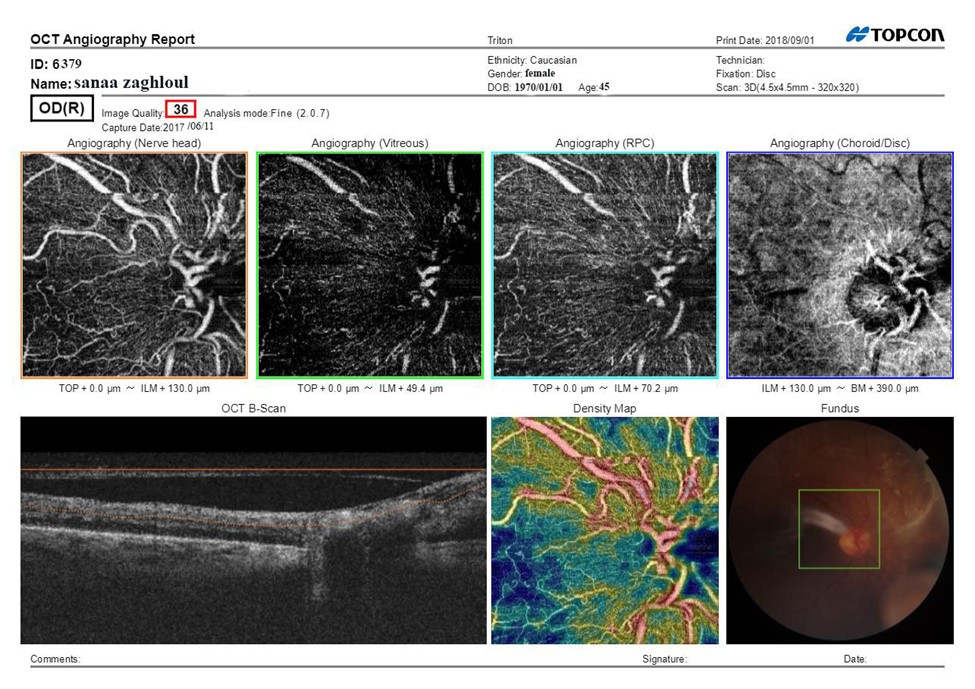
Figure 2B: SS-OCTA scan print out of optic disc after treatment after treatment BCVA: 0.8, CMT: 290 μm, Mean area of NVD (μm):0.55.
The ONH showed general reduction in the visibility of the disc ,areas of capillary drop out and focal areas of vascular attenuation and tufts of neo vascular proliferation with presence of EVP which can be identified as irregular proliferation of small-caliber new vessels before treatment which turned into pruned vascular loops of filamentous new vessels but does not have lesions of EVP after treatment .The vitreous level also shows tuft of neovascularization that decreased after treatment. The RPC of the disc showed areas of poor vascularity. The (choroid/disc) level showed irregularity, attenuation and notching at the chorio-capillary ring. The B-scan showed hyper reflective areas of hemorrhage and fibro vascular proliferation. The vessel density map showed decreased vessel density represented by blue colour and increased vascular density at site of neovascularization represented by red colour. The fundus image showed the location of the scan.
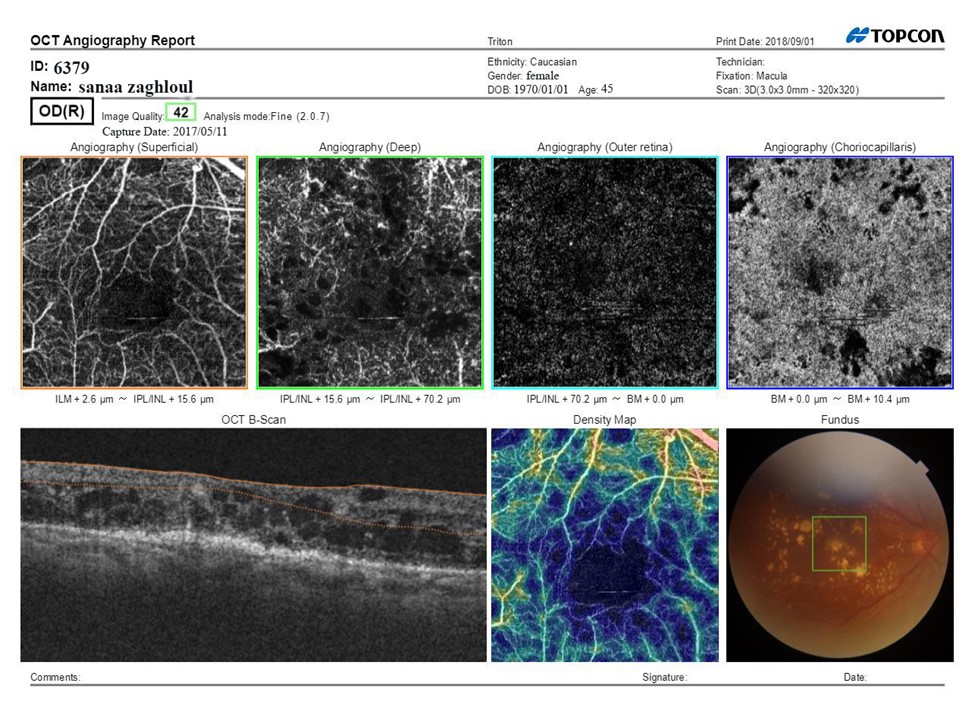
Figure 2C: SS-OCTA scan print out of the macula pretreatment.
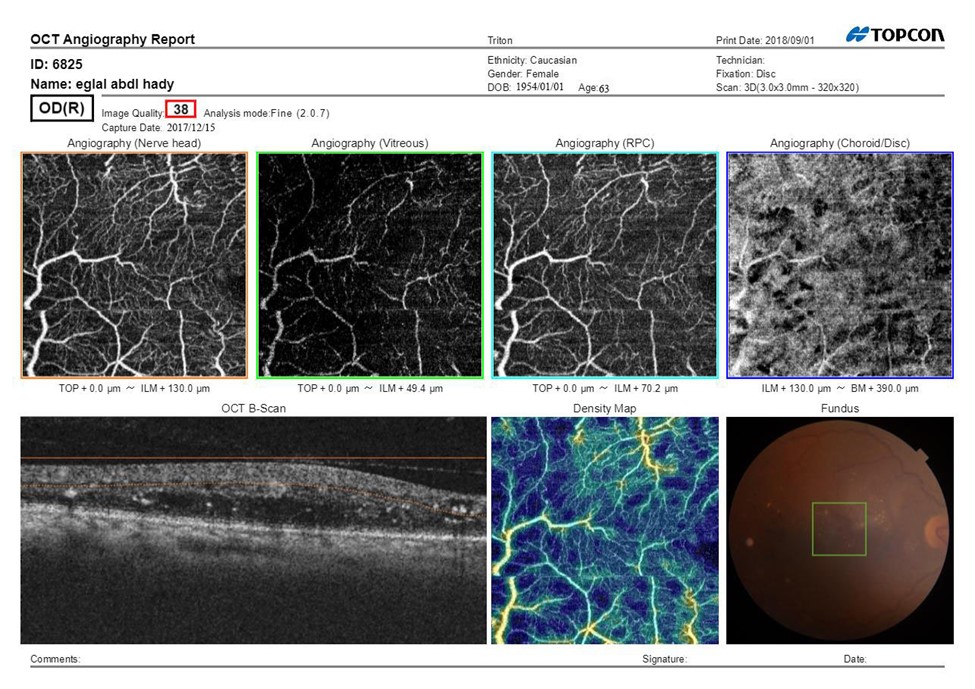
Figure 2D: SS-OCTA scan print out of the macula after treatment.
Discussion
Diabetic retinopathy is considered a major cause of visual impairment and blindness in the world. Over one-third of the world’s 285 million people with DM are estimated to have DR, and one third of these (approximately 31.7 million) have vision-threatening diabetic retinopathy (VTDR) [10]. Pan retinal photocoagulation has been the standard of treatment for PDR for decades. However, intravitreal injection of anti-vascular endothelial growth factor (VEGF) agents have become an alternative for new vessels regression without photocoagulation of peripheral retina [11]. Optical coherence tomography angiography (OCTA) is non-invasive imaging modality for 3-dimensional visualization of retinal and optic nerve capillary networks without the injection of exogenous dyes as compared with fluoresceine angiography (FA) [12], OCTA can monitor different NVD subtypes, their development, and the efficacy of treatment regimens and also can define new vessel morphological details [13]. This study examined a cohort of 50 eyes. Proliferative diabetic changes were detected in the form of NVD in 26 cases (54%) and NVE in 14 cases (28%) and both NVD &NVE in 10 cases (20%). Stanga. et al, had studied 13 eyes with PDR and detected neovessels in 11 eyes and IRMA adjacent to areas of neovascularization in 6 eyes. These results are slightly lower than our study [14,15]. Also Savastano. et al, reported that NVDs observed on FA were also detected on OCTA in all cases (28/28 eyes). Features of NV elsewhere were detected on FFA in 16/28 eyes. Ten of the 16 eyes had signs of NV within the 100 central degrees, and OCTA was able to detect these signs in 9 of the eyes [15,16]. Savastano et al, studied 10 eyes with PDR and concluded that OCTA provides better visualization of NVD than FA which is nearly consistent with our study [16]. On the SS-OCTA scans, areas of multifocal NV were visualized on the total retinal slab enface images. They appeared as irregular, convoluted masses of larger-caliber and smaller-caliber vessels. IRMAs were also apparent on the total retinal slabs and were differentiated as IRMAs rather than NV because of their absence on the corresponding VRI slabs. This is consistent with the findings done by Schaal. et al, [17]. The VRI slabs at 1 month after treatment showed a decrease in the density of smaller-caliber vessels with detectable flow within NV fronds, while showing no significant changes in the larger-caliber vessels that constituted the perimeter and the internal major branches of the NV flow. However, at 3 months, new smaller-caliber neo vascular vessels in some NV fronds were observed to sprout at the margins of the NV. Which is consistent with Russell. et al, [18]. We also have observed that new vessels in patients with PDR could be morphologically divided into two main types: new vessels with Exuberant Vascular Proliferation (EVP) and those without EVP. EVP, which is the intense growth of irregular small-caliber vessels located at the margin of new vessels, likely represents active proliferation, as almost all treatment- na1ve eyes with PDR had lesions with EVP. In contrast, the rate of EVP in new vessels in treated eyes with either PRP & IVI of Ranibizumab has certainly decreased, and the remaining new vessels without EVP in these previously treated eyes had only filamentous vascular loops. These findings are consistent with Ishibazawa. et al, [1]. While the concept of EVP is considered the main tool for the assessment of the activity of the NVD qualitatively, we used NVD areas to compare changes in the NVD before and after treatment quantitatively as suggested by Zhang et al, [19] in their study. We noticed that NVD areas continued to decrease from 1.1± 0.2 mm2 before treatment to 0.5± 0.1 mm2 at 1 month then to 0.3± 0.1 mm2 at 3 months from starting treatment with PRP compared to 1.2± 0.2 mm2 before treatment to 0.6± 0.1 mm2 at 1 month then to 0.4± 0.1 mm2 at 3 months from starting treatment with IVI of Ranbizumab. Zhang et al, has found that before treatment, the mean NVD area was 1.05 ± 0.33 mm, and it decreased to 0.56 ± 0.17 mm after an interval of 7.5 d (p = 0.000). The mean area of the NVD in 14 patients without vitrectomy was 0.22 ± 0.11 mm2 (p = 0.000) at the last visit [19].
These results are slightly different from our results. This difference may be attributed to different version of OCT (Opto Vue, Freemont, California) compared to the version used in our study (Topcon, Tokyo, Japan). This proves that quantitative information on neovascularization can be obtained with OCTA, which may be clinically useful in evaluating the therapeutic effect of the treatment used. According to visual acuity (VA) progression during the whole study comparing group of intra vitreous Ranibizumab with group of PRP , VA at 3 months was good in both groups (approximate mean VA, 0.67±0.1 in cases treated with PRP vs 0.59± 0.2 in cases treated with intra vitreous Ranibizumab).No statistically significant differences were identified between the treatment groups, which is nearly consistent with Gross. et al, that evaluated the efficacy and safety of 0.5-mg intra vitreous ranibizumab vs pan retinal photocoagulation (PRP) over 5 years for PDR showing that although loss to follow-up was relatively high in that study, VA in most eyes that completed follow-up was very good over 5 years [20]. The DRCR Network developed Protocol to compare intra vitreal Ranibizumab with PRP for the treatment of PDR in 394 eyes. At 2 years, the visual outcome of the ranibizumab-treated eyes was non-inferior to the PRP-treated eyes, with a mean visual acuity difference of +2.2 letters in favor of the ranibizumab group. The results of this trial suggest ranibizumab injections are a safe and effective alternative to PRP for the management of PDR [21]. According to central macular thickness (CMT) after treatment in either groups, CMT showed significant decrease across time of treatment, mean (±SD) CMT at diagnosis was 331.5±69. At 1 month of treatment, it was 287.2±55.1, and at 3 months after treatment, it was 241.9±39.4. (p<0.001). No statistically significant differences were identified between the treatment groups, which is nearly consistent with Elman et al, [22]. However, this study had several limitations. First, no ideal scan size could be applied to all lesions. Small scan size (3 mm×3 mm scan) was applied to delineate minute morphology of NVD. But it missed extension of lesions beyond disc margin. Detection of NVE was made possible with larger scan size which mask fine details. Also, it had a relatively small number of patients and a short-term follow.
References
1. Ishibazawa A, Nagaoka T, Takahashi A, et al, 2015. Optical Coherence Tomography Angiography in Diabetic Retinopathy: A Prospective Pilot Study. American Journal of Ophthalmology. 160: 35-44.
2. Vaz-Pereira S, Zarranz-Ventura J, Sim DA, et al. 2016. Optical Coherence Tomography Features Of Active And Inactive Retinal Neovascularization In Proliferative Diabetic Retinopathy. Retina. 36: 1132-1142. Ref.: https://pubmed.ncbi.nlm.nih.gov/26630315/
3. Jia Y, Bailey ST, Hwang TS, et al. 2015. Quantitative optical coherence tomography angiography of vascular abnormalities in the living human eye. 112: 2395-2402. Ref.: https://pubmed.ncbi.nlm.nih.gov/25897021/
4. Huang Y, Zhang Q, Thorell MR, et al. 2014. Swept-Source OCT Angiography of the Retinal Vasculature Using Intensity Differentiation-based Optical Microangiography Algorithms. Ophthalmic Surgery, Lasers and Imaging Retina. 45: 382-389. Ref.: https://pubmed.ncbi.nlm.nih.gov/25230403/
5. El-Sabagh HA, Abdelghaffar W, Labib AM, et al. 2011. Preoperative Intravitreal Bevacizumab Use as an Adjuvant to Diabetic Vitrectomy: Histopathologic Findings and Clinical Implications. Ophthalmology. 118: 636-641. Ref.: https://pubmed.ncbi.nlm.nih.gov/21055812/
6. Rezaei K, Stone T. 2014. Global trends in retina survey. Chicago, IL: American Society of Retina Specialists.
7. Brucker AJ, Qin H, Antoszyk AN, et al. 2009. Observational study of the development of diabetic macular edema following panretinal (scatter) photocoagulation given in 1 or 4 sittings. Arch Ophthalmol. 127: 132-140. Ref.: https://pubmed.ncbi.nlm.nih.gov/19204228/
8. Korobelnik JF, Do DV, Schmidt-Erfurth U, et al. 2014. Intravitreal aflibercept for diabetic macular edema. 121: 2247-2254. Ref.: https://pubmed.ncbi.nlm.nih.gov/25012934/
9. Ferrara N, Damico L, Shams N, et al. 2006. development of ranibizumab, an anti–vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina. 26: 859-870. Ref.: https://pubmed.ncbi.nlm.nih.gov/17031284/
10. Yau J WY, Rogers SL, Kawasaki R, et al. 2012. Global Prevalence and Major Risk Factors of Diabetic Retinopathy. Diabetes Car. 35: 556-564. Ref.: https://pubmed.ncbi.nlm.nih.gov/22301125/
11. Jorge R, Costa RA, Calucci D, et al. 2006. Intravitreal bevacizumab (Avastin) for persistent new vessels in diabetic retinopathy (IBEPE study). Retina. 26: 1006-1013. Ref.: https://pubmed.ncbi.nlm.nih.gov/17151487/
12. Kim DY, Fingler J, Zawadzki RJ, et al. 2013. Optical imaging of the chorioretinal vasculature in the living human eye. Proc Natl Acad Sci U S A. 110: 14354-14359. Ref.: https://pubmed.ncbi.nlm.nih.gov/23918361/
13. Yau J WY, Rogers S L, Kawasaki R, et al. 2012. Global Prevalence and Major Risk Factors of Diabetic Retinopathy. Diabetes Care. 35: 556-564. Ref.: https://pubmed.ncbi.nlm.nih.gov/22301125/
14. De Carlo TE, Romano A, Waheed NK, et al. 2015. A review of optical coherence tomography angiography (OCTA). International Journal of Retina and Vitreous. 1. Ref.: https://pubmed.ncbi.nlm.nih.gov/27847598/
15. Stanga PE Comyn O, Samy A, Qatarneh D, et al. 2016. The Effect of Multispot Laser Panretinal Photocoagulation on Retinal Sensitivity and Driving Eligibility in Patients With Diabetic Retinopathy. JAMA ophthalmology. 134: 666. Ref.: https://pubmed.ncbi.nlm.nih.gov/27077924/
16. Savastano MC, Lumbroso B, Rispoli M 2015. in vivo characterization of retinal vascularization morphology using optical coherence tomography angiography. Retina. 35: 2196-2203. Ref.: https://pubmed.ncbi.nlm.nih.gov/25932558/
17. Schaal KB, Munk MR, Wyssmueller I, et al. 2019. vascular abnormalities in diabetic retinopathy assessed with swept-source optical coherence tomography angiography widefield imaging. Retina. 39: 79-87. Ref.: https://pubmed.ncbi.nlm.nih.gov/29135803/
18. Russell JF, Shi Y, Hinkle JW, et al. 2018. Longitudinal Wide Field Swept Source OCT Angiography of Neovascularization in Proliferative Diabetic Retinopathy After Panretinal Photocoagulation, Ophthalmology Retina. Ref.: https://pubmed.ncbi.nlm.nih.gov/31014688/
19. Zhang X, Wu C, Zhou Lj, et al. 2018. Observation of optic disc neovascularization using OCT angiography in proliferative diabetic retinopathy after intravitreal conbercept injections. Scientific Reports. 8: 1. Ref.: https://pubmed.ncbi.nlm.nih.gov/29507304/
20. Gross JG, Glassman AR, Klein MJ, et al. 2017. Interim Safety Data Comparing Ranibizumab With Panretinal Photocoagulation Among Participants With Proliferative Diabetic Retinopathy. JAMA ophthalmology. 135: 6. Ref.: https://pubmed.ncbi.nlm.nih.gov/28492921/
21. Baker CW, Jiang Y, Stone T. 2016. Recent advancements in diabetic retinopathy treatment from the Diabetic Retinopathy Clinical Research Network. Current Opinion in Ophthalmology. 27: 210-216. Ref.: https://pubmed.ncbi.nlm.nih.gov/26913740/
22. Elman MJ, Bressler NM, Qin H. 2011. Expanded 2-year follow-up of ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 118:609-614.




















