Indexing & Abstracting
Full Text
Research ArticleDOI Number : 10.36811/jca.2020.110005Article Views : 16Article Downloads : 21
First step concerning improvement of arsenic removal by adapted Kanchan filters in the lowlands of Nepal
Barbara Mueller
Bamugeobiochem, Horbenstrasse 4, 8356 Ettenhausen, Switzerland
*Corresponding Author: Barbara Mueller, Bamugeobiochem, Horbenstrasse 4, 8356 Ettenhausen, Switzerland, Email: bar.mueller@unibas.ch
Article Information
Aritcle Type: Research Article
Citation: Barbara Mueller. 2020. First step concerning improvement of arsenic removal by adapted Kanchan filters in the lowlands of Nepal. J Chem Appl. 2: 01-09.
Copyright: This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Copyright © 2020; Barbara Mueller
Publication history:
Received date: 20 January, 2020Accepted date: 27 January, 2020
Published date: 29 January, 2020
Abstract
In the Terai region of Nepal (the southern lowlands of the country) the arsenic concentration of extracted ground water used as drinking water frequently exceeds the actual World Health Organization (WHO) drinking water guideline concentration of 10 μg/L. Single household filters (so called Kanchan filters) are employed to eliminate as from the well water. Being assembled to remove as utilizing zero-valent (ZVI) media, their efficiency was observed to vary to a high degree depending on design, ground water composition and the current operating conditions. Based on these concerns three field campaigns were organized in order to test ground water composition and filter handling on spot. This report depicts for the first time the results of this screening regarding removal efficiencies and clearly disclose future adaptation of the design and enhancement of the Kanchan filters uniquely used in Nepal. Removal efficiency varied between 5.81 % to 97.1 % depending on material, usage and mode of operation. The measurements of improvement include the replacement of nails and sand regularly; increasing the contact time between ground water and nails; preventing the nails from drying in order to maintain oxidizing settings; proper and regularly repeated instructions of the users.
Keywords: Arsenic; Kanchan filters; Removal efficiency
Introduction
The Quaternary sediments of the Himalayan foreland basin in Nepal as well the Bengal fan are widely known as one of the largest fluvial deltas in the world hosting arsenic. This metalloid is contaminating ground water used as drinking water by the inhabitants of the Terai (lowlands of Nepal). Long-term exposure to low concentration of this highly toxic element causes detrimental health effects including skin lesions, cardiovascular and respiratory issues and cancer (see e. g. [1]). Arsenic is undoubtedly geogenic in origin and is therefore assumed to be linked to the elemental weathering of the Himalayan belt [2-5 and references therein].
Heavy monsoon precipitation fills the Terai sediments yearly turning the Quaternary alluvial deposits into an excellent ground water reservoir. The sediments particularly include gravel, sand, silt and clay. Particularly fine-grained sediments like clay were deposited in the inter-fan lowlands together with organic material. Moreover, As is specifically incorporated in fine particles like clay minerals [2-5 and references therein]. Arsenic was long time supposed to be leached out by reductive dissolution of As-rich Fe(III) hydr(oxides) [6]. However, according to other ample investigations, As is most likely concentrated in clayey sediments since it preferentially associated with some specific elements (Al, Na, K, and C) and obviously decoupled from Iron [3-4,7-8]. In addition, Fe(III)hydr(oxides) unlike clay minerals were hardly detected by X-ray analysis in a drill core from the district Nawalparasi [9].
The district Nawalparasi is by far the most affected concerning the arsenic crisis in Nepal. Consequently, this area is the most extensively studied of all Terai provinces regarding local geology and arsenic contaminated ground water. To the east this district is bordered by the Narayani River which is replenishing the unconsolidated Quaternary fluvial deposits. Within areas built up by fine-grained sediments, specifically high concentrations of as are recorded [3,5,10]. In order to remove the toxic agent from the ground water used as drinking water, so called Kanchan filters (single household filter) were installed in the Nepal during the early noughties [11-12]. Built solely and most importantly with locally available material, the filters were designed to remove As by sorption on the surface of Fe(III)(hydr)oxides formed via corrosion of ZVI (Zero Valent Iron) formed in situ from rusty iron nails set in a perforated basin above a thick sand layer intended to remove pathogens by physical straining. The design and operation mode of the filters uses in the lowlands of Nepal are unique in South-East Asia. ZVI was once chosen as adsorbing material as two of the main prerequisites was the local availability and affordability by the residents. As a consequence, no other material has ever been tested in Nepal.
For a detailed discussion of operation of these filters see [2,4-5,11-12 and references therein]. Since the long-term performance of these filters had barely been evaluated, major reservation concerning the removal efficiency arose later [13]. The authors of the last-mentioned study stated that out found that out of 62 tube wells, 41 had arsenic concentration in ground water above Nepal drinking water quality standard value (50 μg/L). From all tested Kanchan filters (KAF) mentioned in this study only 22 reduced As in to a safe level after filtration. Major concerns about the insufficient removal efficiency of Kanchan filters were later expressed by several studies [2,4-5,14-15].
Hence a thorough assessment of the removal mechanisms, efficiency, endurance and material tests clearly seemed warranted. Based on the findings mentioned above, field campaigns were organized in cooperation between CAWST (Center for Affordable Water and Sanitation Technology, Calgary, Canada), ENPHO (Environment and Public Health Organization, Kathmandu, Nepal) and Eawag (Swiss Federal Institute of Aquatic Science and Technology, Dübendorf, Switzerland). The first two sampling campaigns for ground water in Nawalparasi realized in October 2015 (post-monsoon) and in April 2017 (pre-monsoon) demonstrated the influence of the monsoon as well as the influence of the minor element Fe as well as trace elements regarding the removal efficiency of As with Kanchan filters [4-5]. As already mentioned, the design and operation mode of the filters uses in the lowlands of Nepal are unique in South-East Asia. The peculiar geologic situation of Nepal next to the high Himalayas is the cause of a particular composition of the ground water in the Terai [3-5]. Filters being in use for the removal of arsenic therefore have to be evaluated carefully as hardly any literature about the commonplace functioning of the Kanchan filters is available warranting a closer inspection of those filters.
This short communication reveals the results of an initial step of adaption of 30 actually used Kanchan filters and the influence of this adaption on the removal efficiency for the first time ever. Beside replacing the nails and the sand in the lower part of the filter, nails have to be wetted constantly, sufficient contact time between ground water and nails has to be ensured as well as the users have to be instructed on an ongoing base and the efficiency has to be monitored regularly for a number of representative filters.
Materials and Methods
In the vicinity of the villages Manari, Panchanagar, Sukauli and Tilakpur (within a distance of 10 km of Ramgram, the capital of Nawaparasi), ground water trials were sampled from from private, single household hand pumps. On the whole, the depth of the water wells never exceed 25 m. The soil in this depth is generally dominated by clay minerals, quartz, K-feldspar, calcite and dolomite. Three series of 30 employable ground water samples were collected in post-monsoon 2015 and again after the first adaption procedure in pre-monsoon 2018 and 2019. The afore mentioned private pumps were determined representatively according a record provided by ENPHO listing all filtered drinking water exhibiting an as concentration above the Nepal drinking water quality standard value (50μg/L). All samples were later by ICP-MS at Eawag, Dübendorf, Switzerland. For a detailed description of the procedures see [3-5].
Results and Discussion
On the occasion of the three mentioned ground water sampling campaigns 2015, 2018, 2019 beside ground water (raw water from handpumps), samples from partially filtered water just passing the nail bed and the totally filtered drinking water (after passing the sand layer) were collected. For a schematic representation of the Kanchan filters and a typical example of those filters see Figure 1 (Filter SN68).
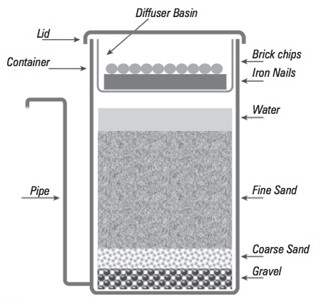
Figure 1a: Diagram of the Kanchan Arsenic Filter, showing the location and arrangement of its components. Source: Ngai et al. [11].

Figure 1b: Filter SN68 in Nawalparasi district, Nepal, October 2018. Photo: B. Mueller.
The concentration of As, Fe and other major and trace elements were determined in these three samples from each filter. The removal efficiency (%) regarding the concentration of as was calculated as follows:
Removal efficiency 1: 100 - (As1*100)/As2
Removal efficiency 2: 100 - (As2*100)/As3
Overall removal efficiency: 100 - (As1*100)/As3
Whereas: As1 = As concentration of ground water, As2 = As concentration of water after passing the nail bed, As3 = As concentration of finally filtered water after passing nail bed and sand layer.
The diameter of these basins, the weight of the nails, the density of the nails and flow rate through the whole filter being given, the contact time with the nails could be calculated. For the sake of clarity, the three different removal efficiency are generally termed removal efficiency 1, removal efficiency 2 and overall removal efficiency. Figure 2 exhibits removal efficiency 1, removal efficiency 2 and overall removal efficiency for all filters evaluated. The efficiencies were calculated for the undisturbed original system viz. as the filters were installed primarily. Samples were taken 2015 and 2018 as some of the sampled filters were not in use anymore or abandoned in 2018.
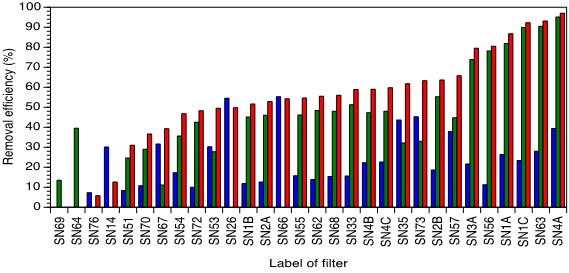
Figure 2: Removal efficiency 1(blue), removal efficiency 2 (green) and overall removal efficiency (red) for all filters evaluated (primary configuration). Negative values were omitted. Data sorted as per highest overall removal efficiency.
The most striking feature is the wide range of the overall removal efficiency ranging from 5.81 % (filter SN76) to 97.1 % (filter SN4A). The omitted negative values of the overall removal efficiencies can be related either to a complete dry nail bed (SN69) or an irregular surface of the nail bed (promoting channels were the ground water can freely pass without contact to the nails) leading to a negative removal efficiency 1. Medium removal efficiency 2 could not outcompete the mentioned irregular removal efficiency 1 due to year-long used sand in the lower sand layer. In general, this sand is hardly ever replaced according testimonies by queried residents. Filter SN76 was determined with a very low overall removal rate owing to the fact that nails and sand were hardly ever replaced. Residence times for filters SN69, SN64 and SN76 were determined to be 19.77 min, 9.69 min and 8.70 min respectively. The best performing filter SN4A was only three years old when probed the first time, hardly ever used, hence maintaining was declared unnecessary by the residents but nails were wet at sampling time after purging the filter thoroughly before sampling. The awesome overall removal rate was as high as 97.1 %. Inspection of the other two removal rates (39.4 % of the nail bed; 95.1 % of the sand layer) clearly indicate the importance concerning the capacity of the fine-grained sand (grain size < 2 mm) to remove exfoliated particles of the from the nails above. Inspection of the calculated contact time with the nails revealed that 31.55 min for filter SN4A which was the highest ever determined so that the extremely high influent as concentration of 735.6 μg/l could be lowered down to 21.65 μg/l despite the low concentration of Fe (1.27 mg/l) [4-5]. In the second-best performer (SN63) with an overall removing efficiency of 93.2 % the nails were lightly covered with water; the filter was used regularly keeping the nails wet and the sand was fresh. Removal efficiencies 1 and 2 were as high as 28.0 % and 90.5 %. The As concentration of the ground water feeding filter SN63 was rather low (158.26 μg/l) and the Fe concentration higher than for SN4A (2.25 mg/l) whereas the residence time was calculated to be 5.74 min. The final concentration of as in drinking water from filter SN63 was as low as 10.8 μg/l (WHO guideline of as concentration in drinking water: 10 μg/l).
Based on these findings a first sand layer above the nail bed was installed in spring 2018 for all 30 filters in order to lower the flow through rate and to increase the contact time with the nails. The sand was separated from the nails by a cloth (cotton-polyester blend) so as to facilitate maintenance. Removal efficiency 1, removal efficiency 2 and overall removal efficiency for all filters sampled immediately after applying cloth and sand are reported in Figure 3. Best performer was again filter SN4A - hardly ever used, nail bed dry at time of inspection leading to a lower removal efficiency 1 compared with the results without the upper sand layer applied (removal rate 1 wet nails and without upper sand bed: 39.4 %; removal rate 1 dry nails with upper sand bed: 17.3 %). The fresh lower sand layer was consistently removing as to a high degree (96.5 %). Filter SN51 showed an increase of the performance of a remarkable degree: The overall removal efficiency without the upper sand bed added only up to 31 % as the nails were dry and glued together leading the water find its way without filtration at the rims of the nail basin. As the plastic bucket containing the nails and the lower sand bed was broken and therefore the filter was leaking, a completely new assessment of this filter on spot was unavoidable. Taking the already utilized nails and using the filter for three days regularly, keeping the nails wet before sampling, while replacing the lower sand layer and applying an upper sand layer lead to the observed increase in performance. The upper sand layer mainly keeps the nails in place, impeding therefore the formation of irregularities in the nail bed itself and lowers the flow through rate leading to an increased contact time. By this means, the initial as concentration of the ground water (239.8 μg) could be lowered to 16.2 μg/l despite a very small concentration of Fe in ground water (0.3 mg/l). Low performance of several filters (e. g. SN70, SN76, SN69) were mainly caused by neglection and poor maintenance. Filter SN64 changed its performance notably after adding an upper sand layer preventing the displacements of the nails which were immersed in water at time of sampling. But even the Fe concentration of the ground water (2.21 mg/l, SN64) was clearly higher than for filter SN51 (0.3 mg/l), the as concentration of the influent water was only lowered from 164.8 μg/l to 51.2 μg/l.
Sampling of all the mentioned filters in spring 2019 revealed that most of the filters equipped with an upper sand layer the performance could be improved (Figure 4). Filter SN1C was working best even with a dry nail bed but black nails at time of inspection. The color is an indication of formation of Fe(III)-oxides removing As to a high degree [14]. According to the users the nail bed of this filter was usually kept wet and interestingly, the Fe concentration of the groundwater was high (6.4 mg/l) whereas the as concentration was only 136.2 μg/l. With an overall removal rate of 95.0 % the as content of the drinking water could be lowered to 6.7 μ/l. Filter SN4A had a lower performance due to an unrequested sort of usage: At the time of inspection the upper concrete plate, sand layer and nail bed were covered with ants. According to the children of the residents they probably purged the filter with a sticky, sugary liquid (e.g. Coke). After dismembering and thoroughly purging the filter with ground water  for 15 min. samples were taken. As the filter was used occasionally throughout the year, the performance was slightly lowered. The efficiency of filter SN64 declined seriously due to poor maintenance, e. g. non-replacement of material like nails and sand. Filter SN51 which was freshly installed in spring 2018 exhibited a lower efficiency owing to aging lower sand layer. Nails from filter SN14 in turn were completely immersed in water. Nails should be kept wet but not immersed as the best removal efficiency was determined in filters with wet nails. Apparently wet nails are oxygenated best, keeping the nail bed wet but not immersed in water.
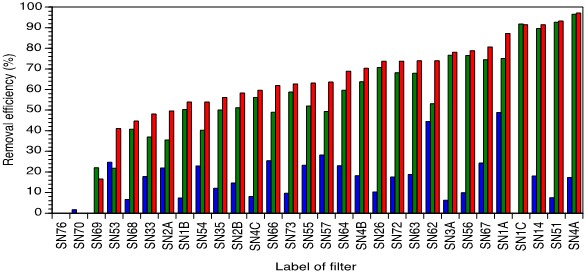
Figure 3: Removal efficiency 1(blue), removal efficiency 2 (green) and overall removal efficiency (red) for all filters evaluated in spring 2018 (secondary configuration including upper sand layer). Negative values were omitted. Data sorted as per highest overall removal efficiency.
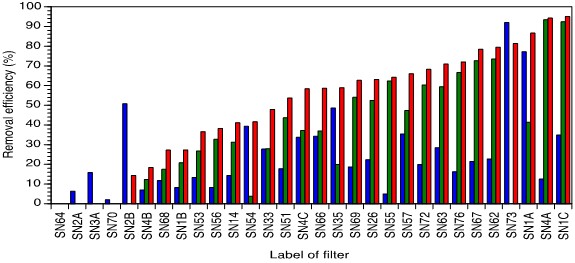
Figure 4: Removal efficiency 1(blue), removal efficiency 2 (green) and overall removal efficiency (red) for all filters evaluated in spring 2019 (secondary configuration including upper sand layer) after one year of regular usage. Negative values were omitted. Data sorted as per highest overall removal efficiency.
A filter with the description SN45 could only be sampled in autumn 2015 and is therefore not included in the data set. The plastic bucket of this filter was leaking later and was not used any longer by the residents of those premises. Ground water feeding this filter had a very high concentration of Fe (5.73 μg/l) but despite this desired fact, the filter did not perform as expected. The overall removal rate was determined to be as low as 70.7 % despite the low concentration of as in ground water (170.2 μg/l). X-ray investigations of nails from some of the mentioned filters herein revealed the presence of siderite (FeCO3) [9]. This mineral is precipitated under still reducing conditions as it contains Fe(II) indicating (i): Oxidation to promote rusting of the nails is not complete and (ii) siderite "seals" the surface of the nails therefore preventing As to be adsorbed on the nails surface or co-precipitating with Fe(III)hydr(oxides) [14,16]. Inspection of the X-ray data clearly indicates that siderite is preferentially formed on nails in contact with ground water containing a high concentration of iron.
Summary, Conclusions, Future improvements
Three different types of Kanchan filters are used in the province Nawalparasi: Plastic round, plastic squares and concrete squares. The brick layer in square filters is commonly replaced by a 2-3 cm think perforated concrete plate keeping the nails in place. Especially concrete square filters are difficult to maintain: As they are fixed on the ground it is a laborious work to replace the lower sand bed. Despite the perforated concrete plate concrete square filters are not performing much better than filters of the other type.
The depicted highly variable removal efficiency clearly depends on several typical prerequisites:
(i) Geological background e. g. Fe and as concentrations of the ground water itself [see 4].
(ii) Condition and aging of the material intended to remove as efficiently: Nails and sand. Both nails and sand have to be changed regularly, especially sand has to be replaced on a yearly base (see filter SN51) in order to maintain its absorbing capacity.
(iii) The nails of the nail bed have to be wet constantly but not immersed in water in order to promote oxidation and formation of Fe(III)hydr(oxides).
(iv) Prevention of formation of holes and dents within the nail bed as the ground water has to be precluded to flow through the nail bed in nail free channels. The ground water has to be poured slowly and carefully in order to prevent displacement of the nails.
(v) Sufficient contact time between ground water and nails. High concentration of As and low concentration of Fe require an increased contact time.
Before sampling users were summoned to fill the filters according their routine manner. This way it was possible to work out if filters were filled, handled and maintained appropriately. It soon became obvious that residents using the filters were not instructed correctly: Zippy pouring of the filters with ground water led to formation of holes in the nail bed, filters were not used on a regular base and therefore the nail bed dried out. The lower sand bed was hardly ever replaced leading to an exhaustion of the absorbing capacity of the aging sand. Moreover, the hand pumps were often placed in direct sunlight or long hoses were installed to transport the ground water from the pump to the filter. The higher the temperature, the less efficient the filters work [4-5]. The applied upper layer of sand is capable of preventing the nails from moving while filling the filter with water. In order to facilitate cleaning of the filter, the upper sand layer was separated by a cloth from the nails [see also: 17-18]. As a consequence, it is imperative to instruct users of the filters correctly and carefully!
Future improvements will include:
(i) Installation of the upper sand bed for all filters in order to prevent the nails from drying and moving while pouring ground water as well as diminishing the flow rate.
(ii) Regulation of the outflow by placing a tap the outlet or by raising the outlet from the plastic bucket to above the level of the nail-bed.
(iii) Replacing nails and sand on a regular basis.
(iv) Proper and regularly repeated instructions of the users.
Acknowledgements
I am grateful to Dr. Stephan Hug and Thomas Rütimann, Eawag, Dübendorf for all analytical work . My great appreciation for all support is also expressed to Tommy Ngai and Candice Young-Rojanschi from CAWST, Calgary, Canada; Bipin Dangol and Hari Boudhatoki ENPHO, Kathmandu, Nepal; Gyan Prakash Yadav, Parasi, Nepal and to Som Rai, Kathmandu, Nepal. This research was supported by various private foundations from Switzerland.
References
1. Abdul KSM, Jayasinghe SS, Chandana EPS, et al. 2015. Arsenic and human health effects: A review. Environ Toxical Pharmacol. 40: 828-846. Ref.: https://bit.ly/2TVX0Zb
2. Mueller B. 2017. Arsenic in groundwater in the southern lowlands of Nepal and its mitigation options: a review. Environ Rev. 25: 296-305. Ref.: https://bit.ly/3aNRwWf
3. Mueller B. 2018. Preliminary trace element analysis of arsenic in Nepalese groundwater may pinpoint its origin. Environ Earth Sci. 77: 35-40. Ref.: https://bit.ly/2sW9PHI
4. Mueller B, Hug SJ. 2018. Climatic variations and de-coupling between arsenic and iron in arsenic contaminated ground water in the lowlands of Nepal. Chemosphere. 210: 347-358. Ref.: https://bit.ly/2TY8vPH
5. Mueller B, Dangol B, Ngai TKK, et al. 2020. Kanchan arsenic filter in the lowlands of Nepal - arsenic load, mode of operation, arsenic removal and future improvements. In print.
6. Nickson RT, McArthur JM, Ravenscroft P, et al. 2000. Mechanism of arsenic release to groundwater, Bangladesh and West Bengal. Appl Geochem 15: 403-413. Ref.: https://bit.ly/3aHgYwG
7. Brikowski TH, Neku A, Shrestha SD, et al. 2014. Hydrologic control of temporal variability in groundwater arsenic on the Ganges floodplain of Nepal. J Hydrol. 518: 342-353. Ref.: https://bit.ly/2TYCo2w
8. Guillot S, Garçon M, Weinman B, et al. 2015. Origin of arsenic in Late Pleistocene to Holocene sediments in the Nawalparasi district (Terai, Nepal). Environ Earth Sci. 74: 2571-2593. Ref.: https://bit.ly/2TZEkI8
9. Zweifel ER. 2018. Arsenic contamination in Nepal: Water treatment issues and geologic origin of the pollution. Master Thesis, Institute of Geography, University of Bern. 67.
10. Diwakar J, Johnston SG, Burton ED, et al. 2015. Arsenic mobilization in an alluvial aquifer of the Terai region, Nepal. J Hydrol, Regional Studies. 4: 59-79. Ref.: https://bit.ly/2RVaCRR
11. Ngai TKK, Murcott SE, Shrestha RR, et al. 2006. Development and dissemination of KanchanTM arsenic filter in rural Nepal. Water Sci Technol. 6: 137-146. Ref.: https://bit.ly/30WrIme
12. Ngai TKK, Shrestha RR, Dangol B, et al. 2007. Design for sustainable development - Household drinking water filter for arsenic and pathogen treatment in Nepal. J Environ Sci Health - A. Toxicol Hazard 42: 1879-1888. Ref.: https://bit.ly/2uxQAVq
13. Singh A, Smith LS, Shrestha S, et al. 2014. Efficacy of arsenic filtration by Kanchan Arsenic Filter in Nepal. J Water Health. 12: 596-599. Ref.: https://bit.ly/2RuqdZy
14. Wenk C, Kaegi R, Hug SJ. 2014. Factors affecting arsenic and uranium removal with zero-valent iron: laboratory tests with Kanchan-type iron nail filter columns with different groundwaters. Environ Chem. 11: 547-557. Ref.: https://bit.ly/2O181oe
15. Chiew H, Sampson ML, Huch S, et al. 2009. Effect of Groundwater Iron and Phosphate on the Efficacy of Arsenic Removal by Iron-Amended BioSand Filters. Environ Sci Technol. 43: 6295-6300. Ref.: https://bit.ly/2U1hZcT
16. Guo H, Stüben D, Berner Z. 2007. Adsorption of arsenic (III) and arsenic(V) from ground water using natural siderite as the adsorbent. J Colloid Interface Sci. 315: 47-53. Ref.: https://bit.ly/36pb4gk
17. Smith K, Li Z, Chen B, et al. 2017. Comparison of sand-based water filters for point-of-use arsenic removal in China. Chemosphere. 168: 155-162. Ref.: https://bit.ly/37v2z4L
18. Smiech KM, Tolsma A, Kovacs T, et al. 2018. Comparing Mixed-Media and Conventional Slow-Sand Filters for Arsenic Removal from Groundwater. Water. 10: 119-132. Ref.: https://bit.ly/3aN8pjN




















