Indexing & Abstracting
Full Text
Case ReportDOI Number : 10.36811/jcri.2021.110026Article Views : 77Article Downloads : 52
Candidemia to Candida pararugosa in a child: first case reported in Morocco
Hami A1*, Elmazgaldi I2, Mouhoub B2, Aziz F2, Elbouchtili E2, Benajiba N3 and Maleb A2
1Laboratory of Parasitology - Mycology, Mohammed VI University Hospital Oujda Faculty of Medicine and Pharmacy, Mohamed the first University, Oujda, Morocco
2Laboratory of Microbiology, Mohammed VI University Hospital Oujda Faculty of Medicine and Pharmacy, Mohamed the first University, Oujda, Morocco
3Department of Pediatrics Mohammed VI University Hospital Oujda Faculty of Medicine and Pharmacy, Mohamed the first University, Oujda, Morocco
*Corresponding Author: Hami Aziza, Laboratory of Parasitology, Mohammed the sixth hospital, Faculty of medicine and pharmacy, Mohamed the first university, Oujda, Morocco, Tel: 00212671079452; Email: azizahami4@gmail.com
Article Information
Aritcle Type: Case Report
Citation: Hami A, Elmazgaldi I, Mouhoub B, et al. 2021. Candidemia to Candida pararugosa in a child: first case reported in Morocco. J Case Rept Img. 3: 57-62.
Copyright: This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Copyright © 2021; Hami Aziza
Publication history:
Received date: 01 October, 2021Accepted date: 06 October, 2021
Published date: 07 October, 2021
Introduction
The genus Candida play an important role in human pathology, which leads scientists to study their virulence and pathogenicity [1]. Some studies have reported that 40% to 50% of infections are caused by Candida species other than Candida albicans. [2-4] Candida pararugosa (C. pararugosa) is a yeast isolated for the first time from human feces. Nakase et al [5] described it in the medical literature in 1999. It was thought that this yeast could be a colonizer of the gastrointestinal tract and oral cavity [6,7] despite the fact that this was isolated from different human anatomical specimens [8], there have been no cases in the literature that have linked its isolation to clinical symptomatology [9]. In Morocco, to our knowledge, no cases of Candida pararugosa candidemia have been reported. In the present observation, we describe the first case of isolation of C. pararugosa from the blood culture of a child with Burkitt lymphoma.
Clinical case
This is a 13 year old child admitted to the emergency room for fever and bone pain (J1). He has no significant pathological history. On clinical examination, the child was conscious, asthenic and pale. Hepatosplenomegaly and axillary, inguinal and retro auricular adenopathies were found. Gingival bleeding was found on examination of the oral cavity. A biological assessment was performed and showed bicytopenia (anemia at 4.5 g/dL and thrombocytopenia at 6000 platelets/mm3 (150-500 x 109 /L)) with lymphocytosis at 17470 lymphocytes/mm3. The blood smear showed 60% blast cells. The myelogram showed Burkitt's lymphoma. An osteo-medullary biopsy and immunophenotyping were performed and the diagnosis of Burkitt lymphoma was retained. Chemotherapy was started at D4. At D14, our proposal was transfused with platelet concentrates due to deep thrombocytopenia with meningeal and alveolar hemorrhage. At D15, he developed a fever of 38.5°C, requiring antibiotic therapy with: Ceftazidime, amikacin, vancomycin and fluconazole with systematic deworming with Metronidazole after 7 days of fever (D22), followed by a combination of Vancomycin, Imipenem and Voriconazole. Because of the persistence of the fever, blood cultures were requested (three vials and at D 24 and D 28 and D 29). They were performed in aerobic vials and incubated in the Becton Dickinson Bactec® FX 400 automated system. After 5 days of incubation (D29, D32 and D33), the blood culture was positive (three positive vials out of three samples).
Microscopic examination after Gram staining showed (Figures 1,2) oval blastospores with mycelial pseudofilaments. Subculture on Sabouraud chloramphenicol medium gave, after 48h incubation at 37°C (D32, D35, D36), smooth, white and flat colonies, 2 to 4 mm in diameter.
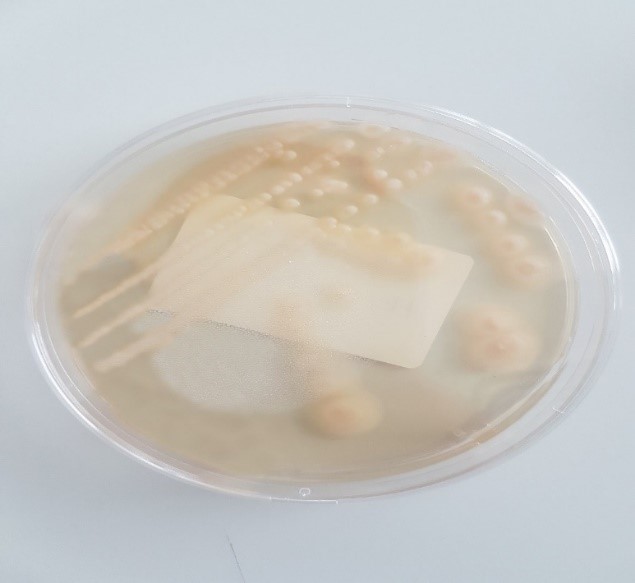
Figure 1: Microscopic examination after Gram staining showing oval blastospores with multilateral buds and mycelial pseudofilaments (×1000).
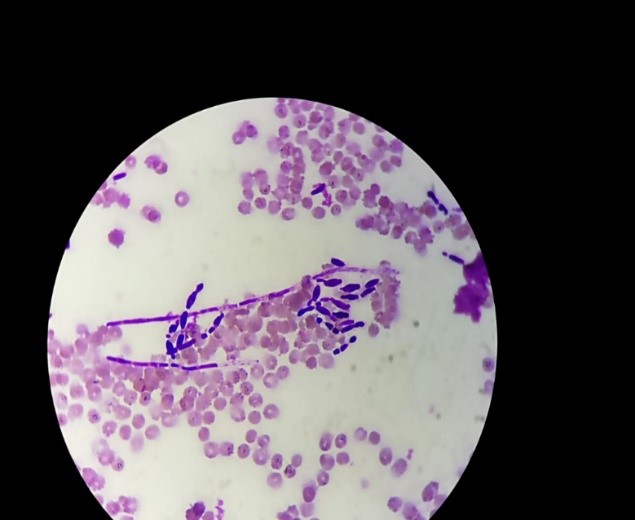
Figure 2: Subculture on Sabouraud chloramphenicol medium.
The identification by biochemical galleries BD PhoenixTM Yeast ID on the BD PhoenixTM 100 automaton revealed the presence of Candida pararugosa with a 99% confidence value. The antifungogram was not performed because it was unavailable. The patient died at D29 of his hospitalization, but the cause of death was not clear, was it candidemia or alteration of the general state secondary to his hematological pathology.
Discussion
Candidemia is the fourth leading cause of sepsis [10,11] with an increasing number of non albicans Candida. The species non albicans Candida was responsible for 54.4% of the candidemia cases [12,13]. Other than Candida albicans, Candida parapsilosis, Candida glabrata, Candida tropicalis, and Candida krusei, rare species have a high mortality rate (57.1%) [14]. These infections are more common in immunocompromised patients, particularly in oncohematology patients [15].
C. pararugosa is a very rare yeast [7]. It was first isolated from human excreta, and after that it was found in the oral cavity of a denture wearer [6] and in the saliva of a patient sarcoma, indicating that it plays a role in the oral microbiota [7]. There is a dearth of knowledge about C. pararugosa natural environment and pathogen role [6]. C. pararugosa has been isolated from a variety of food and environmental sources [16,17] and has been identified as a potential probiotic because of its ability to resist the toxic effects of bile and gastric secretion [18]. C. pararugosa has been isolated from human bronchial washings, blood, urine, and vaginal fluids, according to molecular studies [8-19]. C. pararugosa has also been associated with onychomycosis as a possible pathogen [9]. The analysis of 26 S of activated RNA C. pararugosa to be correctly classified and distinguished from the complex C. rugosa species [20]. Candidemia is defined as an infection proven by the presence of one or more Candida positive blood cultures [21,22]. In our case, this yeast was isolated three times from the blood cultures of a Burkitt lymphoma patient who had been treated with cancer chemotherapy, antibiotics and antiparasitic drugs. Although the exact pathogenicity of this yeast in human pathology is unknown, the isolation of Candida yeast in blood cultures has been linked to a high risk of invasive disease and mortality [9]. This yeast is rare, and it is possible that this is due to the fact that it is under-diagnosed due to the use of methods limited to a simple filamentation test, as in our case , it could have been confused with other Candida species.
The extraction of budding yeasts, with or without filaments, from samples taken from sterile sites can be used to diagnose pathogenic fungi [23-24]. Candida pararugosa blastospores are characterized as oval to long oval elements that occur individually, in pairs, or in short chains to form a pseudohyphae [25]. In our case, the yeasts appear in oval form, 4 µm to 8 µm in diameter, budding, visible on Gram stained smears with the presence of pseudohyphae. The positivity of a sample taken from a normally sterile site is sufficient to diagnose invasive candidiasis. Routinely, repeated blood cultures appear to be the contributory specimen [26]. In the case of our patient, the blood culture was pure and mono-microbial and according to the REMIC criteria for blood cultures, the clinical significance of a microorganism isolated by blood culture is all the more likely that it will be found in successive samples [27]. Yeast was isolated from three blood cultures, two of which were performed 24 hours apart. The patient presented several risk factors for fungemia: immunosuppression, use of broad-spectrum antibiotics, central venous catheters, in particular for the administration of hyper-feeding solutions. The blood contamination could have as an entry point the digestive tract or a vascular catheter [26]. Candida infection is most often linked to endogenous contamination. Indeed, Candida spp are saprophytes of the digestive tract. Under the effect of promoting factors and ecological modifications, they can proliferate in the digestive tract, leading to a colonization phase. The next stage is invasion by translocation during mucosal alteration, with the possibility of distant dissemination (invasive candidiasis, then disseminated candidiasis) [22]. Exogenous contamination occurs more rarely and is caused either by manuporting or by contamination of medical devices (catheters, solutions, etc.) [26]. the patient was transfused several times and received intravenous antibiotic and cancer chemotherapy.
Virulence factors such as adhesion to epithelial and blood cells, where applicable, or yeast are found in direct contact with various blood cells, including red blood cells (Figure 1), biofilm formation, hemolytic activity and production of extracellular hydrolytic enzymes (coagulase, phospholipase and proteinase) [28] facilitate adhesion, infiltration and spread in host tissues, even at elevated temperatures. These factors will promote pathogen resistance to host immunity, such as phagocyte escape and complement mediation. Virulence factors may also include dietary, metabolic, and necrotic factors, as well as morphological variations such as phenotypic change and biofilm formation [29].
However, with the use of specialized fungal media (BD BactecTM Mycosis IC / F Medium) [30], the detection time of candidemia for some strains of Candida can be shortened, which would have prevented the death of the patient. The search for risk factors and the use of predictive scores or indices such as the colonization index can help in the diagnosis of yeast infection. In our patient, a probabilistic antifungal treatment was started with fluconazole and then voriconazole as he was a patient at risk. Several authors have reported that Candida pararugosa has MICs generally elevated on fluconazole [1,8,9] hence the interest of the antifungal chart which is necessary to guide therapy. T confirms the sensitivity of the yeast to the administered antifungal agent and allows the possibility of therapeutic de-escalation, by replacing the initial molecule with one that is as active but less costly or better tolerated [31]. Early initiation of an antifungal treatment is a major prognostic factor for candidemia [32-33].
Conclusion
Candida pararugosa is a rare fungal species. Considered as a commensal yeast of the digestive tract and other tissues, it can become opportunistic in the immunocompromised, especially in oncohematology. Through this observation, we report the first case of C. pararugosa candidemia in Morocco.
References
1. Brandt ME, Lockhart SR. 2012. Recent taxonomic developments with Candida and other opportunistic yeasts. Curr Fungal Infect Rep. 6: 170-177. Ref.: https://pubmed.ncbi.nlm.nih.gov/26526658/ DOI: https://doi.org/10.1007/s12281-012-0094-x
2. García-Rodríguez J, Cantón E, Pemán J. 2013. Incidencia etaria y geográfica y patrón de sensibilidad a los antifúngicos de las especies de Candida causantes de candidemia en la población pediatrica española. Enferm Infecc Microbiol Clin. 31: 363-368.
3. Paula CR, Montelli AC, Ruiz LS. 2007. Infecção hospitalar fúngica: experiência em hospitais públicos de São Paulo. Prática Hosp. 52: 63-66.
4. Ruiz LS, Sugizaki MF, Montelli AC. 2005. Fungemia by yeasts in Brazil: occurrence and phenotypic study of strains isolated at the public hospital, Botucatu, São Paulo. J Mycol Med. 15: 13-21.
5. Suzuki M, Suh SO, Sugita T. 1999. A phylogenetic study on galactose-containing Candida species based on 18S ribosomal DNA sequences. J Gen Appl Microbiol. 45: 229-238. Ref.: https://pubmed.ncbi.nlm.nih.gov/12501365/ DOI: https://doi.org/10.2323/jgam.45.229
6. Giammanco GM, Melilli D, Pizzo G. 2004. Candida pararugosa isolation from the oral cavity of an Italian denture wearer. Res Microbiol. 155: 571-574. Ref.: https://pubmed.ncbi.nlm.nih.gov/15313258/ DOI: https://doi.org/10.1016/j.resmic.2004.04.003
7. Nakagawa Y, Robert V, Kawarazaki J. 2004. Recurrent isolation of an uncommon yeast, Candida pararugosa, from a sarcoma patient. Med Mycol. 42: 267-271. Ref.: https://pubmed.ncbi.nlm.nih.gov/15285058/ DOI: https://doi.org/10.1080/13693780310001597674
8. Paredes K, Sutton DA, Cano J. 2012. Molecular identification and antifungal susceptibility testing of clinical isolates of the Candida rugosa species complex and proposal of the new species Candida neorugosa. J Clin Microbiol. 50: 2397-2403. Ref.: https://pubmed.ncbi.nlm.nih.gov/22553236/ DOI: https://doi.org/10.1128/jcm.00688-12
9. Guy El Helou. 2017. First Reported Bloodstream Infection in an Adult Infectious Disease. Cureus. 9: 1283. Ref.: DOI: https://doi.org/10.7759/cureus.1283
10. Zilberberg MD, Shorr AF, Kollef MH. 2005. Growth and geographic variation in hospitalizations with resistant infections. Emerging Infectious Diseases. 14: 1756-1758. Ref.: https://pubmed.ncbi.nlm.nih.gov/28680771/ DOI: https://doi.org/10.7759/cureus.1283
11. Pfaller MA, Diekema DJ. 2010. Epidemiology of invasive mycoses in North America. Critical Reviews in Microbiology. 36. 1-53. Ref.: https://pubmed.ncbi.nlm.nih.gov/20088682/ DOI: https://doi.org/10.3109/10408410903241444
12. Horn DL, Neofytos D, Anaissie EJ. 2009. Epidemiology and outcomes of candidemia in 2019 patients: data from the prospective antifungal therapy alliance registry. Clinical Infectious Diseases. 48: 1695-1703. Ref.: https://pubmed.ncbi.nlm.nih.gov/19441981/ DOI: https://doi.org/10.1086/599039
13. Arendrup MC, Dzajic E, Jensenetal RH. Epidemiological changes with potential implication for antifungal prescription recommendations for fungaemia: data from a nationwide.
14. Dominique Toubas. 2013. Epidémiologie des candidoses invasives. REVUE FRANCOPHONE DES LABORATOIRES.
15. Bassetti M, Taramasso L, Nicco E. 2011. Epidemiology, species distribution, antifungal susceptibility and outcome of nosocomial candidemia in a tertiary care hospital in Italy. PLoS One. 6: e24198. Ref.: https://pubmed.ncbi.nlm.nih.gov/21935385/ DOI: https://doi.org/10.1371/journal.pone.0024198
16. Jensen SL, Umiker NL, Arneborg N. 2009. Identification and characterization of Dekkera bruxellensis, Candida pararugosa, and Pichia guilliermondii isolated from commercial red wines. Food Microbiol. 26: 915-921. Ref.: https://pubmed.ncbi.nlm.nih.gov/19835781/ DOI: https://doi.org/10.1016/j.fm.2009.06.010
17. Callon C, Duthoit F, Delbès C. 2007. Stability of microbial communities in goat milk during a lactation year: molecular approaches. Syst Appl Microbiol. 30: 547-560. Ref.: https://pubmed.ncbi.nlm.nih.gov/17604934/ DOI: https://doi.org/10.1016/j.syapm.2007.05.004
18. Pennacchia C, Blaiotta G, Pepe O. 2008. Isolation of Saccharomyces cerevisiae strains from different food matrices and their preliminary selection for a potential use as probiotics. J Appl Microbiol. 105: 1919-1928. Ref.: https://pubmed.ncbi.nlm.nih.gov/19120638/ DOI: https://doi.org/10.1111/j.1365-2672.2008.03968.x
19. Taj-Aldeen SJ, Abdul Wahab A, Kolecka A. 2014. Uncommon opportunistic yeast bloodstream infections from Qatar. Med Mycol. 52: 552-556. Ref.: https://pubmed.ncbi.nlm.nih.gov/24934803/ DOI: https://doi.org/10.1093/mmycol/myu016
20. Kurtzman CP, Robnett CJ. 1998. Identification and phylogeny of ascomycetous yeasts from analysis of nuclear large subunit (26S) ribosomal DNA partial sequences, Antonie Van Leeuwenhoek. 73: 331-371. Ref.: https://pubmed.ncbi.nlm.nih.gov/9850420/ DOI: https://doi.org/10.1023/a:1001761008817
21. Ascioglu S, Rex JH, de Pauw B. 2002. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis, 1: 7-14. Ref.: https://pubmed.ncbi.nlm.nih.gov/11731939/ DOI: https://doi.org/10.1086/323335
22. Eggimann P, Garbino J, Pittet D. 2003. Epidemiology of Candida species infections in critically ill nonimmunosuppressed patients. Lancet Infect Dis. 11: 685-702. Ref.: https://pubmed.ncbi.nlm.nih.gov/14592598/
23. Chabasse D, Robert R, Marot A. 2006. Candida pathogènes. Paris, Lavoisier.
24. Rispail P. 2005. Epidémiologie et diagnostic biologique des candidoses muqueuses et cutanéo-phanériennes. Dermatologie.
25. Takashi N, Kazuo K, Yoshimura F. 1978. Candida pararugosa, a asporogenous new species of yeast. J Gen App Microbiol. 24: 17-25.
26. T Clavier, A Lefèvre-Scelles, B Veber. 2014. Les infections à levures en réanimation, Le Congrès Médecins. Conférence d’Actualisation.
27. Société française de microbiologie, Société française de mycologie médicale, Société française de parasitologie. Référentiel en microbiologie médicale. Paris: SFM. 2015.
28. Sachin C. Deorukhkar, Santosh S. 2014. Interdisciplinary Perspectives on Infectious Diseases. 7.
29. Khan M, Ahmad I, Aqil F. 2010. Virulence and pathogenicity of fungal pathogens with special reference to Candida albicans. In: Combating Fungal Infections. Ahmas I, Owais M, Shahid M, Aqil F (Ed.), Springer, Heidelberg, Germany. 21-45.
30. Horvath LL, George BJ, Murray CK. 2004. Direct comparison of the BACTEC 9240 and BacT/ALERT 3D automated blood culture systems for candida growth detection. J Clin Microbiol. 1: 115-118. Ref.: https://pubmed.ncbi.nlm.nih.gov/14715740/ DOI: https://doi.org/10.1128/jcm.42.1.115-118.2004
31. A Paugam. 2010. Trends in antifungal susceptibility test methods, La Lettre de l’Infectiologue.
32. Grim SA, Berger K, Teng C. 2012. Timing of susceptibility-based antifungal drug administration in patients with Candida bloodstream infection: correlation with outcomes. J Antimicrob Chemother. 3: 707-714. Ref.: https://pubmed.ncbi.nlm.nih.gov/22184469/ DOI: https://doi.org/10.1093/jac/dkr511
33. Kollef M, Micek S, Hampton N. 2012. Septic shock attributed to Candida infection: importance of empiric therapy and source control. Clin Infect Dis. 12: 1739-1746. Ref.: https://pubmed.ncbi.nlm.nih.gov/22423135/ DOI: https://doi.org/10.1093/cid/cis305




















