Indexing & Abstracting
Full Text
Research ArticleDOI Number : 10.36811/jvsr.2020.110010Article Views : 7Article Downloads : 43
Effect of antioxidants on lysozyme and complement activities in heat-stressed rabbits
Doaa Sayed Abdel-Hady1, Yasser Kamal Badawi2, Amany Sayed Maghraby3 and Mahmoud Abdel-Latif4*
1Biotechnology Department, Animal Production Research Institute, Agriculture Research Centre, Beni-Suef, Egypt
2Biotechnology Department, Animal Production Research Institute, Agriculture Research Centre, Giza, Egypt
3Department of Therapeutic Chemistry, The Division of Pharmaceutical and Drug Industries Research, The Group of Immune and Biomarkers for Infection, (The Centre of Excellence for Advances Sciences). The National Research Centre, El Buhouth St., Dokki, Cairo, Egypt
4Immunity division, Zoology Department, Faculty of Science, Beni-Suef University, Beni-Suef, Egypt
*Corresponding Author: Mahmoud Abdel-Latif, Department of Zoology, Faculty of Science, Beni-Suef University, Beni-Suef, Egypt, Email: mahmoud_1232000@yahoo.com
Article Information
Aritcle Type: Research Article
Citation: Mahmoud Abdel-Latif, Doaa Sayed Abdel-Hady, Yasser Kamal Badawi, et al. 2020. Effect of antioxidants on lysozyme and complement activities in heat-stressed rabbits. J Veterina Sci Res. 2: 01-18.
Copyright: This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Copyright © 2020; Mahmoud Abdel-Latif
Publication history:
Received date: 03 January, 2020Accepted date: 09 January, 2020
Published date: 13 January, 2020
Abstract
The negative influence of heat stress (HS) on rabbit farms cannot be ignored. The current study aims to detect the effect of Moringa oleifera leaf extract (MOE), Vitamin C (Vit C) and sodium bicarbonate (NaHCO3) on HS-induced changes in the complement, lysozyme, and antioxidant activities. A total of 45 growing male NZW rabbits were divided into five groups: Negative control rabbits were not exposed to HS (group 1), while HS groups (2-5) were exposed to high temperatures (39?). Group 2 was exposed to HS but non-treated while the rest of groups were treated with MOE, Vit C and NaHCO3, respectively. Expression of lysozyme mRNA, as well as the activities of lysozyme, complement and antioxidants, were assayed. Serum IL-1β was also assayed using ELISA. Furthermore, the histopathological changes of liver and kidney, blood urea nitrogen (BUN), body weight gain (BWG), and mortality rate of experimental animals, were investigated. Our data showed a decrease in lysozyme, antioxidant activities, and BWG versus increases in complement activity, IL-1β level, and BUN in HS. Histological investigation showed an increase in inflammatory reactions. MOE, Vit C and NaHCO3 could alleviate HS effects through increased activities of lysozyme and antioxidants versus decreased complement activity leading to improved health status.
Keywords: Heat stress; Lysozyme; Complement; Antioxidant; Immunity
Introduction
Rabbit production is one of the fast growing enterprises because of the rabbit characteristics like fast production, rapid growth rate, early maturity, efficient feed utilization, and high quality nutritious meat with low cholesterol level. Exposure to elevated ambient temperatures in developing countries showed restrictions in rabbit production as a result of decreased growth rate, body weight gain, reduced fertility, and disruptions to various physiological signs leading to higher mortality rates [1]. Environmental-induced HS takes great attention because of its destructive impacts on domestic animals [2]. HS was well-defined as the stress generated inside the body which made the animals disabled to adjust their heat homeostasis passively [1, 3]. It was considered as the most important risk facing rabbit development and extension in hot climatic regions [4]. In Egypt, the HS period extends more than 6 months during which production is affected adversely [5]. It induced physiological stress in rabbit and subsequent production losses [6, 7]. It has been obvious that there is an association between stress, animal behavior, the neuroendocrine and immune systems [8, 9]. Acute and chronic stress exerted its cytotoxic influence via induction of apoptosis and inhibition of natural cell-mediated immunity [10].
No significant differences between two rabbit genders after exposure to HS were observed [1]. However, male rabbit was usually used in the experimental study because of its high susceptibility for greater expression of HSP70 after induction with heat stress than female [11]. Lysozyme and the complement system are interesting indicators for non-specific immunity functions [12]. Lysozyme is a vital element of the innate immune response as it participates in inactivation of invading microbes and was generally affected by environmental stress [13]. It is located in lysosomes of neutrophils, monocytes, macrophages, and glandular cells, and released in the blood from these cells [14]. Overexpression of the native defense protein lysozyme could protect against acute oxidative stress [15]. It was observed that the temperature fluctuations affected lysozyme activity [16]. Exposure to HS led to an increase in extracellular Hsp70, which was established as a potent activator of complement and IL-1β [17]. Moreover, the synthesis of complement 3 could be up-regulated by IL-1β and IL-6, while complement 4 was influenced by IFN-γ [18, 19]. Elevated ambient temperature induced the free radicals that led to damaging oxidative stress to different organs like liver and kidney [20, 21]. Thus, HS increased the lipid peroxidation and decreased the antioxidants level [22]. This was associated with increased mortality rates [2]. MOE, Vit C and NaHCO3 were found to improve oxidative stress and heat tolerance by their immunomodulatory activities [23-25].
The aim of the present study was to evaluate the alleviating potential of these diet supplements on HS–induced alterations through testing the activities of lysozyme, complement, and antioxidants.
Methods
Experimental animals and housing
The experiment was carried out using artificial climate chambers at Sids agriculture research station, Beba, Beni-Suef, Egypt. A 12-h light, 12-h dark (12L:12D) daily schedule was used. Forty-five New Zealand White (NZW) male rabbits (age was one month and a half and average body weight was 1.29 Kg ± 0.064) were used (from 15 March to 27 April 2016) as experimental animals in this investigation and were housed individually in galvanized wired cages (35 × 35 × 60 cm). Rabbits were adapted for about 10 days before the onset of the experiment. During this period, rabbits were maintained at 23? and relative humidity 60%, given ad libitum access to feed and water and observed for any undercurrent infections. The formulation and chemical analysis of the basal diet (bd) were constructed as shown in Table 1 [26].
|
Table 1: Formulation and calculated chemical composition of the basal diet. |
|
|
Item |
Percentage(%) |
|
Ingredients |
|
|
Wheat bran |
25.5 |
|
Barely |
23 |
|
Soybean meal |
21.5 |
|
Wheat straw |
19.5 |
|
Yellow corn |
7.5 |
|
Limestone |
1.5 |
|
Di-calcium phosphate |
0.5 |
|
NaCl |
0.3 |
|
Vitamins & Minerals |
0.3 |
|
Dl-methionine |
0.2 |
|
Anti-coccidia |
0.1 |
|
Anti-fungal |
0.1 |
|
Total |
100 |
|
Chemical composition |
|
|
Dry matter |
89 |
|
Crude protein |
17.06 |
|
Digestible energy (Kcal Kg-1) |
2605 |
|
Crude fiber |
13.12 |
|
Calcium |
0.91 |
|
Phosphorus |
0.64 |
|
Lysine |
0.87 |
|
Methionine + cystine |
0.69 |
To confirm that particular temperature and humidity were maintained in each climatic chamber, Hydro thermographs TES-1361C (TES electrical electronic Corp, Taipei, Taiwan) were used to record temperature and relative humidity every 5 min for each chamber. From these data, mean daily air temperature and relative humidity were calculated. Temperature– humidity index (THI) was estimated according to the previously developed equation for rabbits [6]: THI = db (in °C) - {(0.31 - 0.31 RH) × [db (in °C) - 14.4]}, where db: is dry bulb temperature in degrees Celsius and RH is the relative humidity percentage/100. Calculated THI values were subsequently classified as follows: <27.8 = absence of heat stress, 27.8–28.9 = moderate heat stress, 28.9–30.0 = severe heat stress, and >30.0 = extremely severe heat stress.
Preparation of MOE
Moringa leaves were obtained from the Egyptian scientific association of Moringa, at National Research Center (Dokki, Giza, Egypt). The leaves were harvested, air-dried under shade until the moisture of collected leaves reached 10%. Dry leaves were finely milled, sieved (1 mm mesh) and stored in a well tight polyethylene bags at room temperature 25°C. The aqueous extract was prepared by mixing 100 g dried powdered leaves of M. oleifera with 100 mL of boiling water for 5 minutes [27]. Mixture was then filtered twice through a 2 μm pore sterile filter paper. The aqueous extract stock solution (100 mg/mL) was freshly prepared for each set of experiments.
Experimental design
Rabbits were randomly allocated into 5 groups (9 per group). The first group acted as a negative control where the rabbits were housed at a constant 23°C with 60% relative humidity. The second group acted as HS group and housed in temperatures that fluctuated between 28°C and 39°C on a daily basis with relative humidity 60%. Similar to the second group, the third, fourth and fifth groups were heat stressed but orally administered daily for 6 weeks with 100, 200 and 300 mg of MOE [28], Vit C [29] and NaHCO3 [23] / Kg body weight in water, respectively. The daily temperature regimen was described in detail (Figure 1). These conditions imitate the daily summer condition of Egypt.
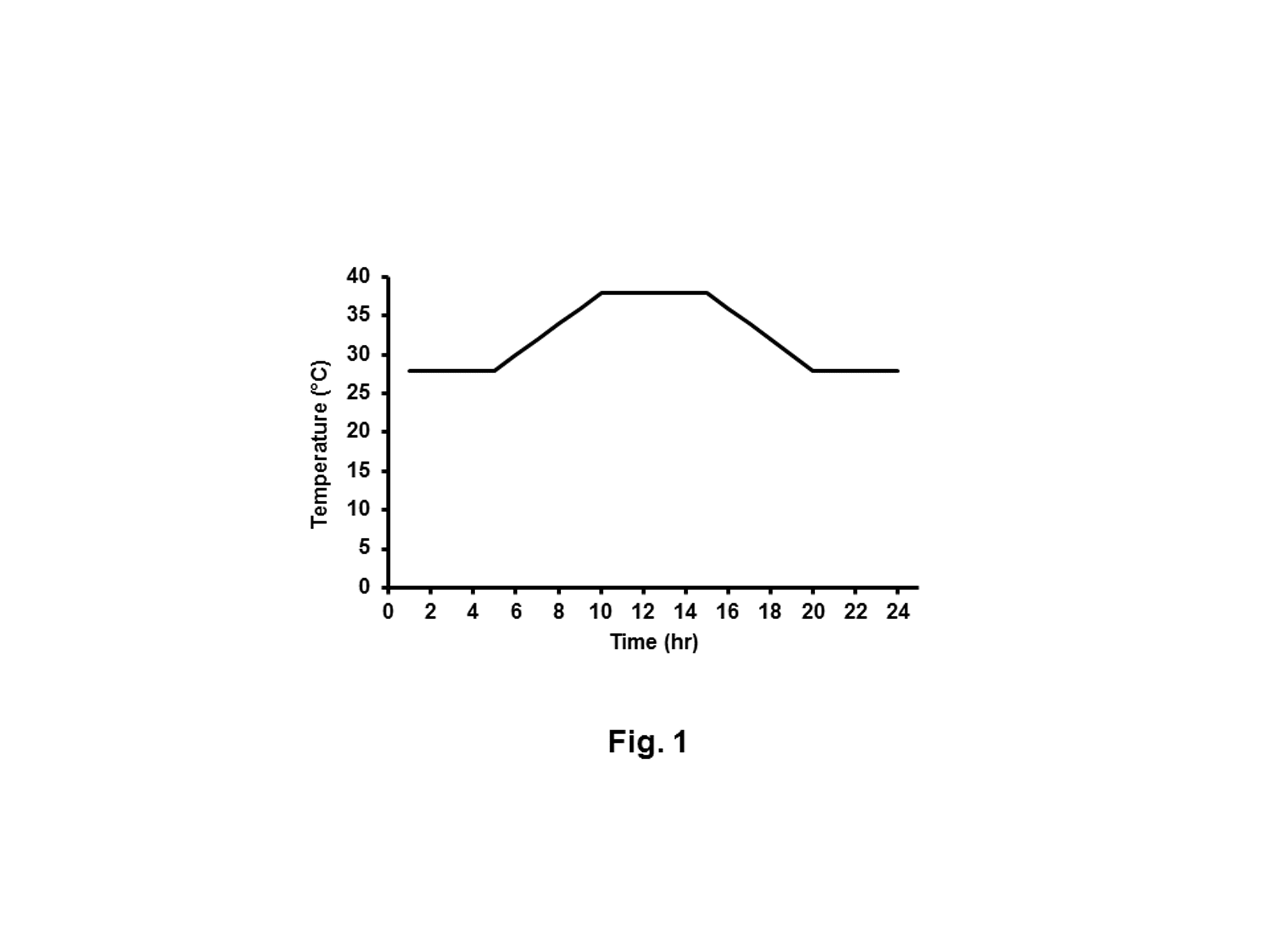
Figure 1: The record of temperature cycle during a single day of heat stress. HS rabbits were subjected to heating regimen that imitates the summer climate of Egypt. From 5:00 to 10:00 hours, the temperature was gradually increased from 28°C to 38°C and maintained at 38°C for 5 h and then gradually decreased to 28°C.
Serum and tissue samples preparation
After rabbits were euthanized according to the ethical committee of Beni-Suef University, 5 mL of the blood samples were collected into a sterile vacutainer tube and allowed to coagulate for serum preparation by centrifugation at 3500 x g for 15 min, transferred into sterilized tubes and stored at -20°C. Parts of liver and kidney tissues were homogenized in phosphate buffer saline (PBS) using Potter–Elvehjem homogenizer (Braun, Melsungen, Germany) with a loose-fitting Teflon pestle at 1000 x g with eight up-and-down strokes. After filtration, the homogenate was centrifuged at 600 x g for 10 min at 4°C in a Beckman TJ-6 centrifuge (Beckman Instruments, Munich, Germany). The clear supernatants were separated and used for analysis. Other parts of liver and kidney tissues were fixed in 10% buffered formalin (Sigma, St. Louis, USA), dehydrated in alcohol series and then embedded in paraffin wax for histological examination. Pieces of liver and kidney were kept at -70°C until they were used for RNA extraction and quantitative RT-PCR analysis.
Quantitative RT - PCR
The protocol was performed as described [30]. Total RNA was extracted from parts of the liver and kidney using spin or vacuum (SV) total RNA isolation system (Promega, Madison, WI, USA). Contaminating genomic DNA was digested with the DNA-free™ kit (Applied Biosystems, Darmstadt, Germany), before cDNA was synthesized using a reverse transcription kit (Stratagene, San Diego, CA, USA). RT-PCR was performed in a TaqMan7500 (Applied Biosystems) according to the manufacturer’s instructions. After that, 5 μL of the reverse transcription products were then amplified using the QuantiTect™ SYBR® Green kit (Qiagen Hilden, Germany) under the following conditions: denaturation at 95°C for 10 min; followed by 30 cycles of 95°C for 10 s, 60°C for 35 s, and 72°C for 30 s; followed by 5 min of extension at 72°C. Forward and reverse primer sequences used for rabbit lysozyme and β- actin are shown in Table 2. All PCR reactions yielded only a single product of the expected size as detected by melting point analysis and gel electrophoresis. Quantitative evaluation was performed with Taqman7500 system software (Applied Biosystems). Expression of lysozyme was normalized to that of β- actin.
Lysozyme activity
The level of serum lysozyme activity was measured by agarose gel lyses assay as previously described [31]. Briefly, lyso plates were prepared by dissolving 1% agarose in 0.06 M PBS at pH 6.3 and 500 mg of bacterial uniform suspension (Micrococcus lysocleikticus) in 5ml saline was added to 1L of agarose. Plates were poured then 25μL of serum samples and standard lysozyme was put in each well. After 18 hours, the cleared zone diameter was measured to both standard lysozyme and serum samples and concentration was estimated. The diameter of the sample was plotted against the standard for obtaining the lysozyme activity in μg/mL.
|
Table 2: Primer sequences for quantitative detection of lysozyme mRNA expression. |
|
Target gene Primer sequence |
|
Lysozyme F :5’-ACGACACTGGCAACATGAGG-3′ |
|
R:5′- ATTCCAACATCACGCAGACC-3′ |
|
β-actin F :5’- GAAATCGTGCGTGACATTAAG -3' |
|
R:5′- CTAGAAGCATTTGCGGTGGAC -3' |
Hemolytic Complement activity
Fifty% hemolytic complement activity (CH50) in rabbit sera was determined as previously described [32]. The amount of complement activity was determined by examining the capacity of various dilutions of test serum to lyse antibody-coated sheep red blood cells. The degree of hemolysis was quantified by measuring the absorbance of the hemoglobin released into the supernatant at 540nm.
BUN
BUN was calorimetrically determined in animal sera by Spectrum Diagnostics kit (Obour city, Egypt) and UV 160 spectrophotometer (Shimadzu, Kyoto, Japan).
ELISA
The level of IL - 1β (pg/ mL) in sera was assayed using ELISA kit (My Bio-Source, San Diego, CA, USA). The procedures were carried out according to the instructions provided with the kits.
Measurement of oxidative stress in liver and kidney tissues
Malondialdehyde (MDA) concentration was determined as an indicator of lipid peroxidation in liver and kidney tissues as previously described [33]. MDA content was measured using a commercial kit (Biodiagnostic, Giza, Egypt). MDA was expressed as nmol/g tissue. The absorbance was measured by using a spectrophotometer at wavelength 534 nm. Superoxide dismutase (SOD) activity was estimated as previously described [34] using a commercial kit (Biodiagnostic). The absorbance was measured at 560 nm for 5 min at 25?C using a spectrophotometer. The level of SOD activity was expressed as units/g tissue. The concentration of reduced glutathione (GSH) in liver and kidney homogenates was measured as previously described [35] using a commercial kit (Biodiagnostic). The absorbance was measured at 405 nm. The GSH concentration was expressed as mmol/g tissue. The level of catalase activity was measured (Biodiagnostic) based on the colorimetric method as previously described [36]. The absorbance was measured at 510 nm and the level of catalase activity was expressed as U/g tissue.
Growth performance and mortality
Initial body and final body weights were individually taken at the beginning and at the end of the experiment, respectively. BWG was calculated as previously described [37]. Mortality was recorded throughout the study period, and mortality rate (MR) was calculated according to the following equation.
Statistical analysis
Data were presented as mean ± SD. Statistical analysis was performed using SPSS version 20 for Windows (SPSS Inc., Chicago, IL). The comparisons between negative control and HS or HS and HS-treated rabbit groups were determined by one-way analysis of variance (ANOVA) LSD-t-test. A simple linear correlation analysis was processed by Pearson’s method to measure the degree of dependency between variables using MedCalc statistical program (Ostend, Belgium). Statistical significance was assumed at p< 0.05.
Results
Meteorological parameters
The mean of THI values obtained in this work were 21.97 on the negative control and 31.10 on the remaining groups, indicating absence of heat stress in the first group (negative control) and exposure of the remaining rabbit groups to extremely severe heat stress (>30.0) during which several of the studied traits were adversely affected [6].
Effect of MOE, Vit C and NaHCO3 on HS-induced histopathological changes
For the liver tissue, the negative group showed normal structures (Figure 2a). While the HS group showed obvious histopathological alterations (Figure 2b). Besides to the existence of pyknotic nuclei in hepatocytes, there were also mononuclear leucocytic inflammatory cell infiltrations in the portal area. Moreover, the liver showed vacuolar degeneration of hepatocytes along with dilated sinusoids (Figure 2c). On the other hand, treatment with MOE, Vit C, and NaHCO3 (Figures 2 d, e &f) caused marked amelioration and restoration to the nearly normal structure of hepatic tissue except that the infiltrated inflammatory leucocytic cells still existed after Vit C treatment. The dilated central vein and sinusoids, as well as vacuolated hepatocytes and hemorrhage, were absent.
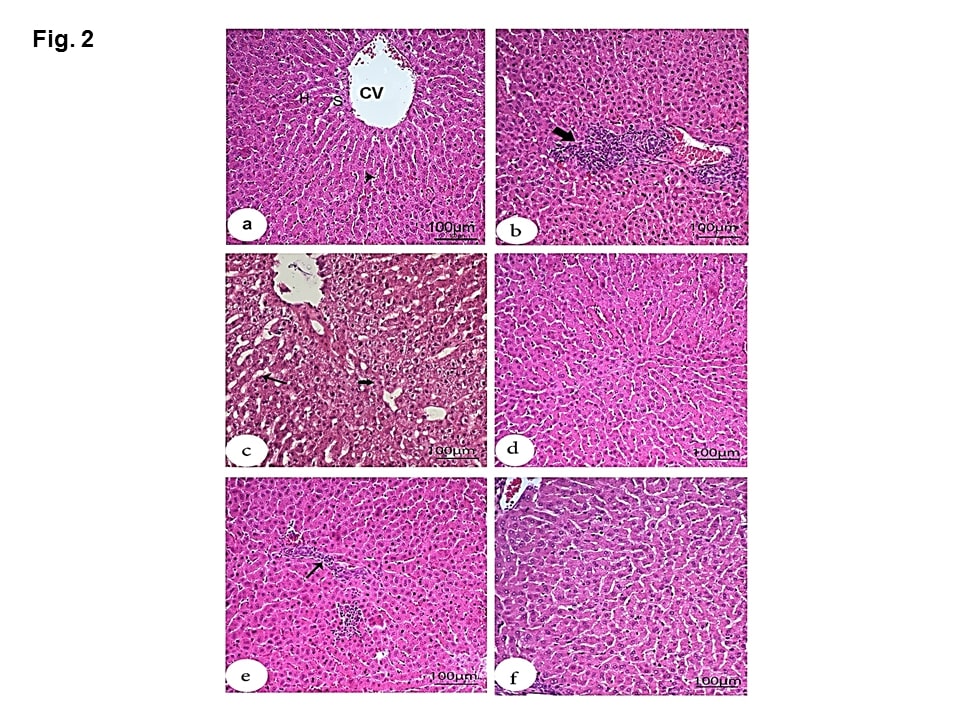
Figure 2: Effect of MOE, Vit C and NaHCO3 on HS-induced histopathological changes in liver tissue showing control group (a) with normal structures including central vein (CV), hepatocytes (H), sinusoids (S) and Kupffer cells (arrowheads); HS group (b & c) with mononuclear cell infiltration in the portal area (large thick arrow), hepatocytes with pyknotic nuclei, vacuolar degeneration (small thick arrow) and dilated sinusoids (thin arrow); treatments with MOE and NaHCO3 showing great recovery and restoration to nearly normal structure of hepatic tissue (d and f), respectively) and treatment with Vit C (e) showing moderate recovery except the presence of inflammatory leucocyte cell infiltration in the parenchyma (thin arrow). (H & E, scale bar 100).
Microscopic examination of the kidney sections of the negative group showed normal structures including renal corpuscles with normal glomerulus, Bowman’s space and normal tubules (Figure 3a). HS group showed enlargement of some renal corpuscles with atrophy of the glomerular tufts. Bowman’s spaces were wide, while the lumina contained amorphous material. (Figure 3b). Degeneration of the epithelial cells lining the renal tubules and mononuclear cell infiltration in the intertubular space were detected (Figure 3 c). MOE, Vit C and NaHCO3 restored the normal structure except that Vit C showed slightly widened Bowman’s spaces (Figures 3 d, e &f).
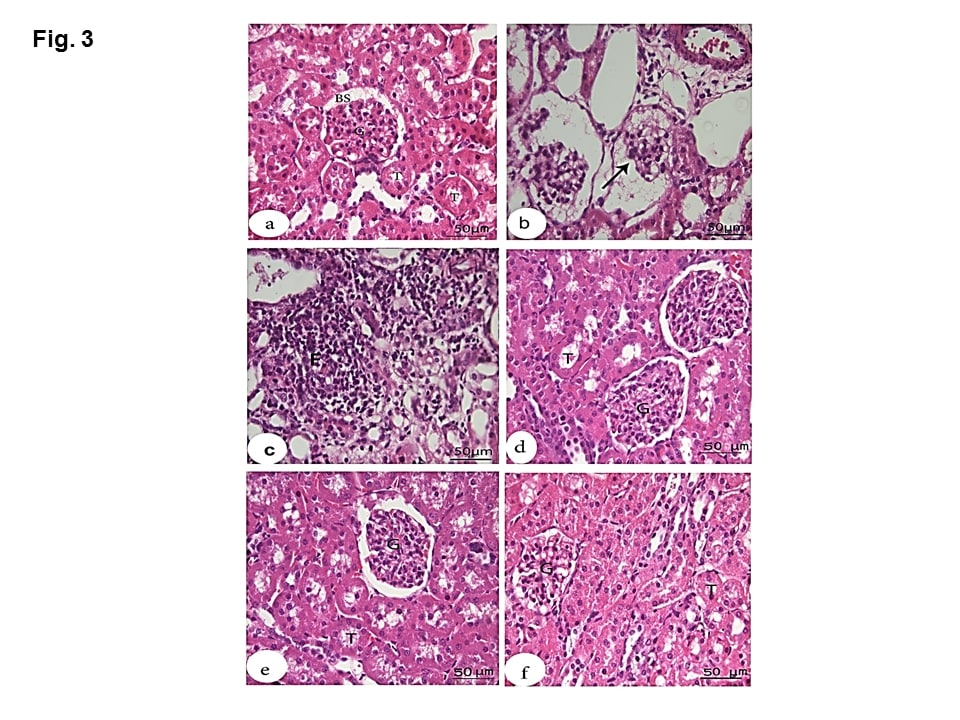
Figure 3: Effect of MOE, Vit C and NaHCO3 on HS-induced histopathological changes in kidney tissue showing control group (a) with normal structures including renal corpuscles with normal glomerulus (G), normal Bowman’s space (BS) and normal tubules (T); HS group (b & c) with atrophied degenerated glomerular tufts (arrow), intertubular mononuclear cell infiltration (F) and degeneration of the epithelial cells lining the renal tubules. Treatments with MOE NaHCO3 and Vit C (d, e and f, respectively) showed great recovery and restoration of the nearly normal structure of the renal corpuscles and the renal tubules. (H & E, scale bar 50).
Lysozyme mRNA expression in liver and kidney tissues
The expression of lysozyme mRNA in liver or kidney was significantly decreased (P< 0.001 and 0.01, respectively) in the HS group compared to the negative control group (Figure 4A). The expression of liver lysozyme mRNA was significantly increased (P< 0.01, 0.05 and 0.01) in HS-treated rabbits with MOE, Vit C and NaHCO3, respectively versus HS group. In the kidney, the expression of lysosome mRNA was significantly increased (P< 0.05) in HS-treated rabbits with NaHCO3 with respect to HS group, while its expression in HS- groups treated by MOE and Vit C did not change significantly.
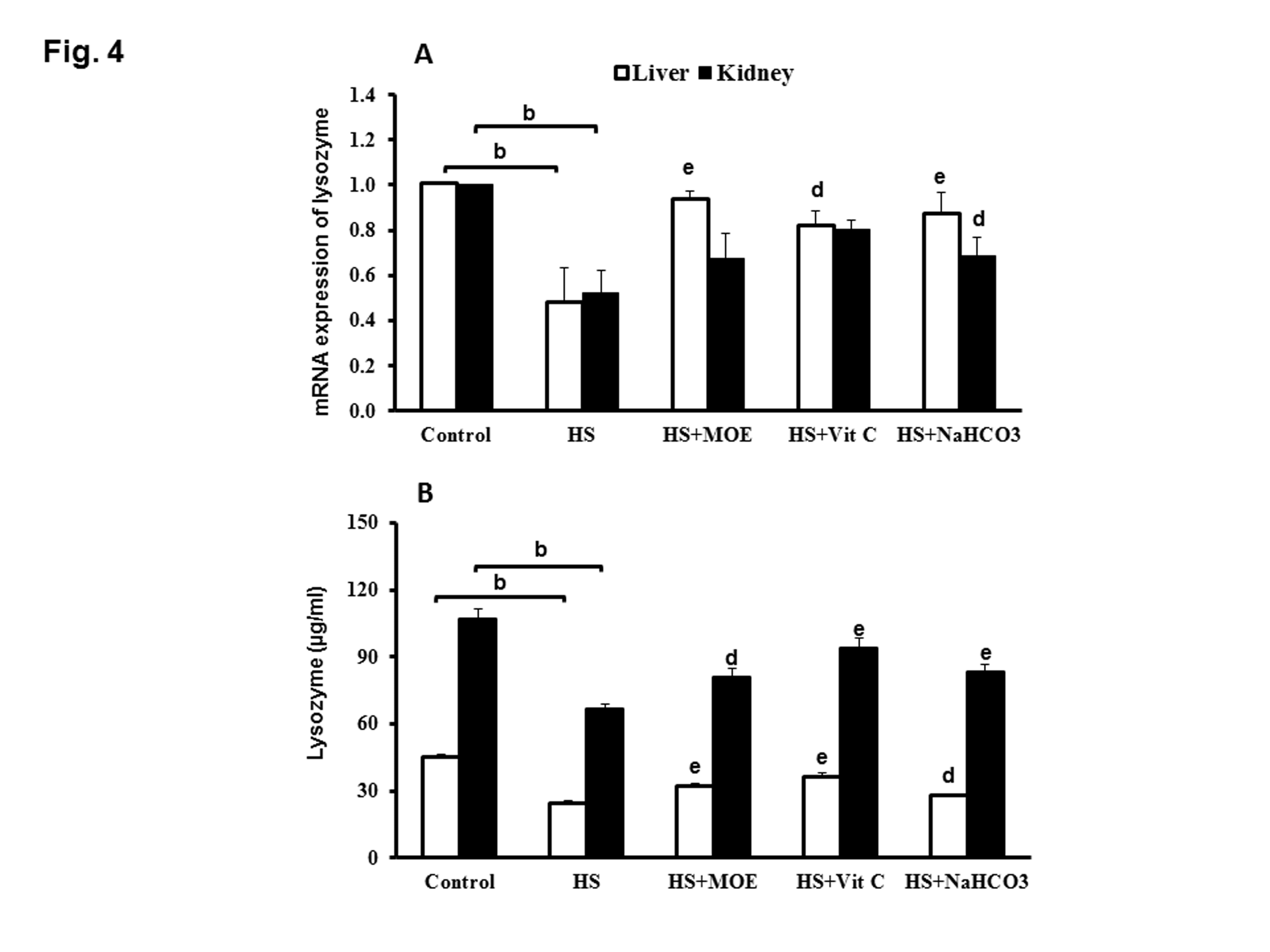
Figure 4: Effect of MOE, Vit C and NaHCO3 on HS-induced decrease in mRNA expression of lysozyme (A) and lysozyme activity (B) in both of liver and kidney tissues. HS decreased its expression and activity in both tissues versus the control group. All treatments reversed the levels of expression and activity to normal levels. Data are expressed as mean± SE. "b" represents the degree of significance (p<0.01) between HS and control groups, while "d & e" represent the degree of significance (p<0.05 and p<0.01, respectively) between treated groups and HS group.
Lysozyme activity in serum and tissue samples
The level of lysozyme activity in liver or kidney tissues from HS group was significantly decreased (P<0.001 and 0.01) as compared with negative control group. Interestingly, the level of liver and kidney lysozyme activities in HS-treated groups with MOE, Vit C or NaHCO3 were significantly increased versus HS group (Figure 4B). Serum lysozyme activity in HS group was significantly decreased (P<0.01) versus negative control group, while, lysozyme activities in sera from HS treated groups with MOE or Vit C or NaHCO3 were significantly increased (P<0.01) with respect to HS group (Figure 5A).
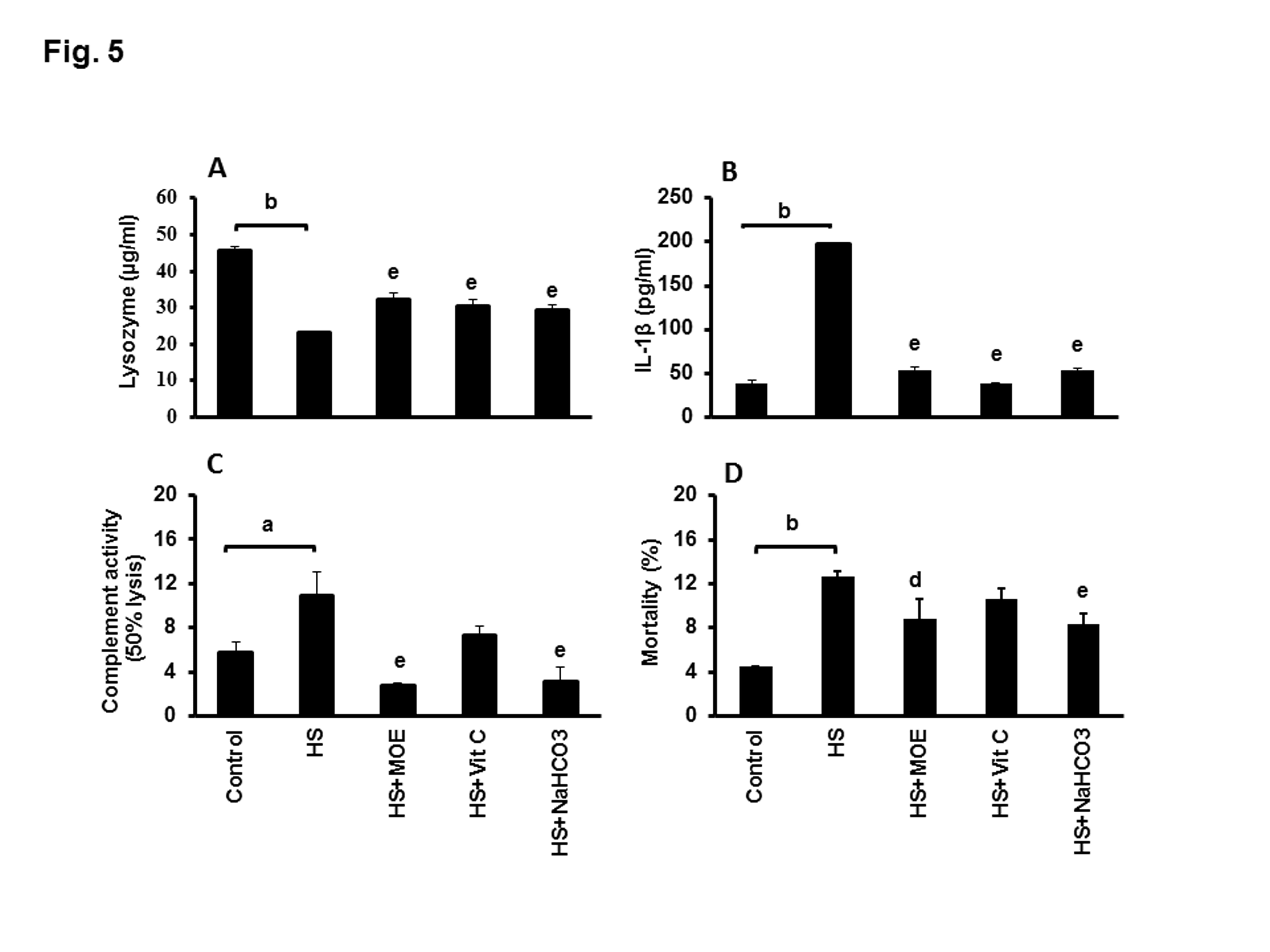
Figure 5: Effect of MOE, Vit C and NaHCO3 on HS-induced decrease of serum lysozyme activity (A) and HS-induced increases in IL-1β (B), complement (C) and mortality (D). Treatments with MOE, Vit C and NaHCO3 reversed both of lysozyme activity and IL-1β to normal levels, while only MOE and NaHCO3 could reverse the levels of complement and mortality to normal levels. Data are expressed as mean± SE. "a & b" represent the degree of significance (p<0.05 and p<0.01, respectively) between HS and control groups, while "d & e" represented the degree of significance (p<0.05 and p<0.01, respectively) between treated groups and HS group.
Complement activity and IL-1β level
IL-1 β level showed a significant upregulation (p< 0.01) in HS versus control group (Figure 5B). On the contrary, the levels of IL-1β were significantly reversed to normal (P<0.01) in HS-treated groups with MOE, Vit C or NaHCO3 versus HS. The level of complement activity showed a significant increase (P<0.05) in HS group versus control group (Figure 5C). In contrast, the level was significantly decreased (P< 0.01) in HS-treated with MOE or NaHCO3 in comparison to HS group.
Assessment of correlation between lysozyme and complement levels in serum
Table 3 illustrated a negative correlation between the levels of lysozyme and complement activities in sera of control and HS groups (-0.6, -0.9; P< 0.05) respectively, while Vit C-treated group showed a positive correlation (1; P< 0.01).
Effect of MOE, Vit C and NaHCO3 on HS-induced mortality rate
HS group showed a significant increase (P<0.01) in mortality rate with respect to the control group (Figure 5D). MOE and NaHCO3 treated groups showed a significant decrease (P< 0.05 and 0.01, respectively) in the mortality rate.
Levels of MDA, GSH, SOD, and catalase in liver and kidney tissues
The MDA level in liver and kidney tissues from HS group was significantly increased (P< 0.01) in comparison to the negative control group. The MDA level was significantly reversed to the normal level (P<0.01) in HS-treated groups with MOE, Vit C or NaHCO3 in comparison to HS group in both tissues (Figure 6). In liver and kidney tissues, the level of GSH, as well as SOD and CAT enzyme activities in HS group, were significantly decreased (P<0.01) versus the control group. Their levels were significantly increased in both tissues of MOE, Vit C and NaHCO3 treated groups as compared to HS.
|
Table 3: Correlation analysis (r) between complement and lysozyme activities in serum. |
|||
|
Lysozyme |
|||
|
r |
p value |
||
|
Complement |
Control |
-0.6 |
0.040* |
|
HS |
-0.9 |
0.012* |
|
|
HS + MOE |
-0.5 |
0.543 |
|
|
HS + Vit C |
1 |
0.001** |
|
|
HS +NaHCO3 |
-0.5 |
0.653 |
|
|
*, < 0.05 ;**, < 0.01 |
|||
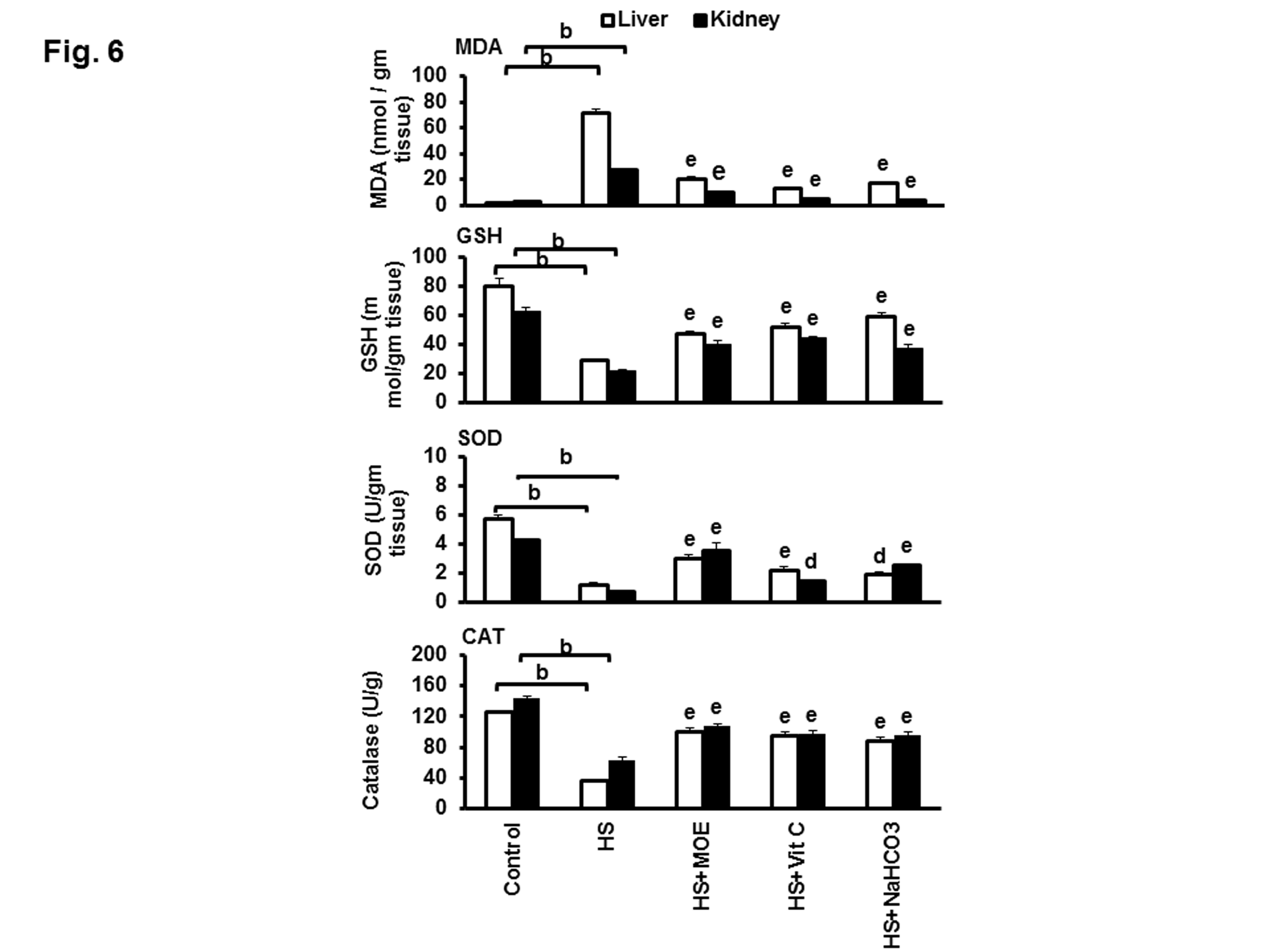
Figure 6: Effect of MOE, Vit C and NaHCO3 on HS-induced oxidative stress in the liver and kidney tissues. The figure shows the variations in the levels of MDA &GSH and activities of SOD and CAT. HS increased MDA and decreased GSH, SOD, and CAT in both tissues with respect to negative control. Treatments reversed these levels to normal values with respect to both negative and HS groups. Data are expressed as mean± SE. "b" represents the degree of significance (p<0.01) between HS and control groups, while "d & e" represent the degree of significance (p<0.05 and p<0.01, respectively) between treated groups and HS group.
Levels of BUN and growth performance
BUN was significantly (p< 0.001) increased in HS versus control group (Figure 7A). All treatments could decrease such levels close to normal with respect to both control and HS groups. Thus MOE, Vit C and NaHCO3 could reverse kidney dysfunctions caused by HS in rabbits. HS caused alterations in the growth performance of rabbits because BWG was decreased (p< 0.001) in comparison to the control group (Figure 7B). Treatment with NaHCO3 showed a significant (p< 0.05) protection against BWG decrease.
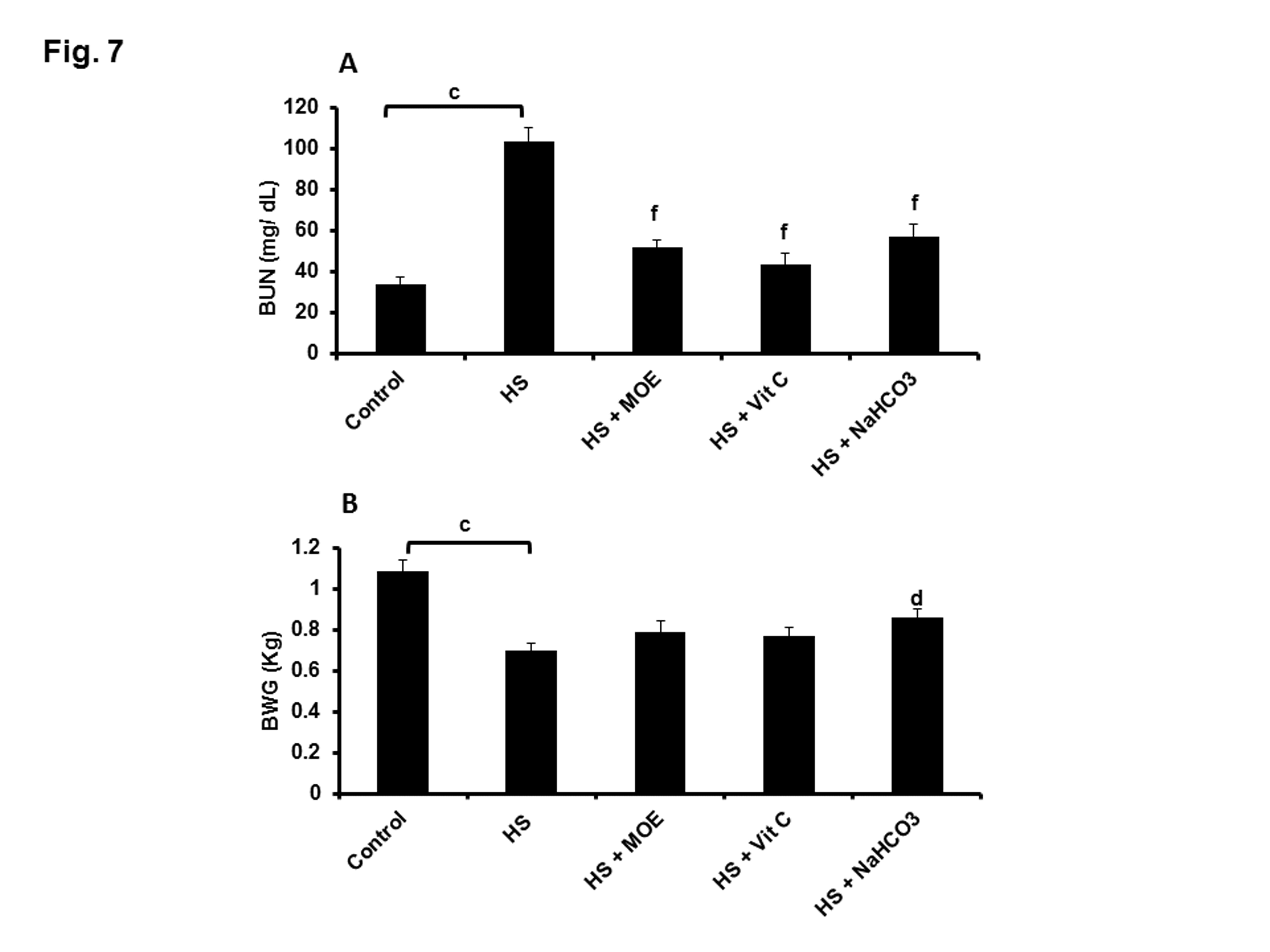
Figure 7: Effect of MOE, Vit C and NaHCO3 on HS-induced increase and decrease in BUN (A) and BWG (B), respectively. All treatments could decrease BUN significantly, while only NaHCO3 could protect against BWG reduction. Data are expressed as mean± SE. "c" represents the degree of significance (p<0.001) between HS and control groups, while "d & f" represent the degree of significance (p<0.05 and p<0.001, respectively) between treated groups and HS group.
Discussion
Lysozyme is a vital element as it possesses antibacterial and anti-inflammatory properties [38]. In the current study, HS decreased lysozyme expression and activity levels compared with control group. Indeed, the negative effect of HS on lysozyme level could be referred to the increase in corticosterone concentrations [7,39]. An increase in glucocorticoid as a result of HS could deactivate lysozyme gene transcription and subsequently produced a strong reduction of the circulating lysozyme concentrations [40]. Its activity was found dependent on several factors like type, pattern, strength, and duration of stress [41]. Generally, under short-time acute stress, the activity of lysozyme was increased to elevate the resistance, while under long-time chronic stress, lysozyme activity was reduced [42].
Treatments with different doses (100, 200, and 300 mg/ Kg, respectively) of MOE, Vit C and NaHCO3 was based on the previous reports about the minimum doses required to achieve anti-oxidant effects [23, 28, 29]. These treatment doses could increase lysozyme on both mRNA and protein levels. This indicated the sensitivity of lysozyme to cellular stress. The influence of MOE on lysozyme may be related to rich quercetin, β-sitosterol, zeatin, caffeoylquinic acid and kaempferol constituents of M. oleifera leaves which might stimulate the immune function [43]. The supplementation with Vit C increased the activities of lysozyme because Vit C enhances the innate immunity [44, 45]. Similarly, aerosolizing HCO3- onto cystic fibrosis airways in vivo increased lysozyme activity as one of the effective arms for bacterial killing [46]. Indeed, NaHCO3 was capable of enhancing the bacterial killing through increased pH.
In the current study, HS upregulated the level of the complement activity (classical pathway) in blood because of increased extracellular HSP70 expression [7,17,47]. These extracellular HSPs played a vital role in the activation of Toll-like receptors (TLRs) on macrophages and neutrophils which, in turn, secreted inflammatory cytokines like IL-1β [48]. IL-1β had a positive effect on serum complement factor 3 synthesis [18, 19]. However, complement activity was claimed to decrease under heat stress [49]. MOE and NaHCO3 could reduce the complement activity level in blood indicating the diagnostic potential of complement in cellular stress. This was explained as MOE constituents are β-sitosterol and quercetin which were reported to have anti-inflammatory and immunomodulating properties [50, 51]. This was also shown in MOE–induced reduction of IL-1β level. Similarly, M. oleifera seeds showed the same anti-inflammatory effect on acetic acid-induced acute colitis in rats [52]. Treatment with Vit C increased complement activity but decreased IL-1β and MDA levels. It was previously found that the deficiency of Vit C was related to decreased complement activity and increased upregulation of proinflammatory cytokines like IL-1β [53]. Vit C could obviously increase the level of complement activity in rabbits because of its ability to induce the synthesis of complement protein C1r [54]. Furthermore, the inhibitory effect of NaHCO3 on complement activity in HS was referred to the alkalosis of blood [55]. In addition, low pH possibly activated complement through activation of the alternative pathway (AP) and through pH-sensitive cross-talk between the coagulation and complement systems [56].
Investigations for HS group revealed a negative correlation between lysozyme and hemolytic complement activity. This was also confirmed on different sheep breeds as the breed that showed the highest serum lysozyme concentration was associated with low complement activity [57]. Moreover, principal component analysis demonstrated that lysozyme and complement activities are negatively related [13]. The possible explanation could be sought in a functional feedback mechanism which compensates the low complement activity with high serum lysozyme concentrations serving as a protective barrier. However, treatment with Vit C could preserve the synergistic effects for both complement and lysozyme activities.
The mortality rate increased in HS group as previously reported [58, 59]. This was referred to several factors as a result of chronic HS exposure including highly elevated glucocorticoid levels which stimulated catabolism of skeletal muscle as an emergent source of energy. This led to reduced survival probability. Moreover, it has been evident that the exposure of cells to overheating could stimulate variations in nuclear and cytoskeletal structures and increased apoptosis [10]. Severe heating damaged many organs such as liver as a result of encouraged production of reactive oxygen species (ROS) [60]. As proved by our result of histopathology showing inhibition of pyknotic nuclei, administration of MOE, Vit C and NaHCO3 to HS-exposed rabbits could defend against histopathological alterations, oxidative stress, and death. Overexpression of lysozyme could protect against acute oxidative stress as the antioxidant feature of lysozymes was partly mediated by a drop in ROS levels [15]. MOE, Vit C and NaHCO3 could reduce lipid peroxidation by quenching peroxide radicals [60-63]. Chronic HS led to a decrease in the level of antioxidant enzymes (SOD and catalase), while treatment could reverse it. MOE was found to induce antioxidant effect through activation of peroxisome proliferator-activated receptor alpha (PPARα), while Vit C was found to induce heme oxygenase expression and production [64,65]. NaHCO3 was found tin increase the ratio of dissolved CO2/ dissolved O2 leading to activation of antioxidant enzymes [66]. Treatment with NaHCO3 was the only one successful for protection against body weight loss. This confirms the previous study performed for testing the effect of NaHCO3 to improve the health status of animals received cisplatin [67]. This may refer to the acid-base balance after treatment leading to enhancement of metabolic pathways [68]. Generally, treatment of HS-induced changes downregulated inflammatory reactions (decreased IL-1β and complement activation) but upregulated antioxidant activities (increased GSH, SOD, CAT, lysozyme activities and decreased MDA). Treatment with MOE, Vit C and NaHCO3 could also improve the kidney functions through reduction of blood urea nitrogen. MOE and Vit C actions on the renal artery were to reduce the resistance index and decrease the renal ROS [69,70]. For NaHCO3, urinary alkalinization and prevention of metabolic acidosis is beneficiary for kidney function [71].
Conclusion
Supplementation of MOE, Vit C and NaHCO3 to rabbits exposed to HS generally improved the antioxidant status, lysozyme and complement activities leading to survival. In addition, lysozyme and complement activities were found critical for alleviating heat stress-induced cellular alterations.
Acknowledgments
The authors are grateful for Prof. Dr. Nadia Mostafa (Department of Zoology, Faculty of Science, Beni-Suef University, Beni-Suef, Egypt) for her great efforts in translating the images of histopathology. The authors are also grateful for Prof. Dr. Thabet Sakran for his support and encouragement to finish the work.
Authors’ contributions
MAL designed and supervised the study, carried out statistical analysis and wrote the manuscript. DSAH carried out the laboratory work. YKD co supervised the study and wrote the experimental layout. ASM approved the experimental protocol, read and corrected the manuscript. All authors read and approve the final manuscript.
Ethical approval
All protocols were approved by the animal ethics committee within the Beni-Suef University in accordance with the international guiding principles for biomedical research involving animals, as issued by the council for international organizations of medical sciences.
References
1. Ondruska L, Rafay J, Okab AB, et al. 2011. Influence of elevated ambient temperature upon some physiological measurements of New Zealand White rabbits. Veterinární medicína. 56: 180-186.
2. Belhadj Slimen I, Najar T, Ghram A, et al. 2016. Heat stress effects on livestock: molecular, cellular and metabolic aspects, a review. Journal of Animal Physiology and Animal Nutrition. 100: 401-412. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/26250521
3. Askar AA, Ismail EI. 2012. Impact of heat stress exposure on some reproductive and physiological traits of rabbit does. Egyptian journal of animal production. 49: 151-159. Ref.: https://bit.ly/35zBewh
4. El Saidy NR, Fatma F, El-Sayed A, et al. 2016. Evaluation of using honey, cool water and levamisole against heat stress on different traits of rabbits under Egyptian summer conditions. World's Veterinary Journal. 6: 10-18. Ref.: https://bit.ly/2N3t0Xb
5. Hashem NM, AbdEl-Hady A, Hassan O. 2013. Effect of vitamin E or propolis supplementation on semen quality, oxidative status and hemato-biochemical changes of rabbit bucks during hot season. Livestock Science. 157: 520-526. Ref.: https://bit.ly/305gf3C
6. Marai IF, Ayyat MS, Abd el-Monem UM. 2001. Growth performance and reproductive traits at first parity of New zealand white female rabbits as affected by heat stress and its alleviation under Egyptian conditions. Tropical Animal Health and Production. 33: 451- 462. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/11770200
7. Abdel-Latif M, Sakran T, Badawi YK, et al. 2018. Influence of Moringa oleifera extract, vitamin C, and sodium bicarbonate on heat stress-induced HSP70 expression and cellular immune response in rabbits. Cell Stress and Chaperones. 23: 975-984. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/29728855
8. Straub RH, Miller LE, Schölmerich J, et al. 2000. Cytokines and hormones as possible links between endocrinosenescence and immunosenescence. Journal of Neuroimmunology. 109: 10-15. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/10969175
9. Won E, Kim YK. 2016. Stress, the autonomic nervous system, and the immune-kynurenine pathway in the etiology of depression. Current Neuropharmacology. 14: 665-673. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/27640517
10. Katschinski DM, Boos K, Schindler SG, et al. 2000. Pivotal role of reactive oxygen species as intracellular mediators of hyperthermia-induced apoptosis. The Journal of Biological Chemistry. 275: 21094-21098. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/10781588
11. Morrow G, Hightower LE, Tanguay RM. 2015. Small heat shock proteins: big folding machines. Cell Stress Chaperones. 20: 207-212. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/25536931
12. Dabbou S, Rotolo L, Kovitvadhi A, et al. 2016. Rabbit dietary supplementation with pale purple coneflower. 1. Effects on the reproductive performance and immune parameters of does. Animal. 10: 1101-1109. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/26763800
13. Simide R, Richard S, Prévot-D'Alvise N, et al. 2016. Assessment of the accuracy of physiological blood indicators for the evaluation of stress, health status and welfare in Siberian sturgeon (Acipenser baerii) subject to chronic heat stress and dietary supplementation. International Aquatic Research. 8: 121-135. Ref.: https://bit.ly/2N2vrZU
14. Maes M, Twisk FN, Kubera M, et al. 2012. Evidence for inflammation and activation of cell-mediated immunity in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): increased interleukin-1, tumor necrosis factor-α, PMN-elastase, lysozyme and neopterin. Journal of Affective Disorders. 136: 933-939. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/21975140
15. Liu H, Zheng F, Cao Q, et al. 2006. Amelioration of oxidant stress by the defensin lysozyme. American Journal of Physiology-Endocrinology and Metabolism. 290: 824-832. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/16317028
16. Tokarz-Deptu?a B, Nied?wiedzka-Rystwej P, Adamiak M, et al. 2015. Natural immunity factors in Polish mixed breed rabbits. Polish Journal of Veterinary Sciences. 18: 19-28. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/25928906
17. Prohászka Z, Singh M, Nagy K, et al. 2002. Heat shock protein 70 is a potent activator of the human complement system. Cell stress chaperones. 7: 17-22. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/11892984
18. B?ogowski W, Budkowska M, Sa?ata D, et al. 2013. Clinical analysis of selected complement-derived molecules in human adipose tissue. Journal of Translational Medicine. 11: 11. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/23302473
19. Makki K, Froguel P, Wolowczuk I. 2013. Adipose tissue in obesity related inflammation and insulin resistance: cells, cytokines, and chemokines. ISRN Inflammation. 2013: 139239. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/24455420
20. Del Vesco AP, Gasparino E, Grieser DO, et al. 2015. Effects of methionine supplementation on the expression of protein deposition-related gene in acute heat stress-exposed broilers. PLoS One. 10: 0115821. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/25714089
21. Guo L, Li R, Zhang YF, et al. 2018. A comparison of two sources of methionine supplemented at different levels on heat shock protein 70 expression and oxidative stress product of Peking ducks subjected to heat stress. Journal of Animal Physiology and Animal Nutrition. 102: 147-154. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/28503893
22. Türk G, Çeriba?? AO, ?im?ek ÜG, et al. 2016. Dietary rosemary oil alleviates heat stress-induced structural and functional damage through lipid peroxidation in the testes of growing Japanese quail. Animal Reproduction and Science. 164: 133-143. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/26656503
23. Peart DJ, Kirk RJ, Hillman AR, et al. 2013. The physiological stress response to high-intensity sprint exercise following the ingestion of sodium bicarbonate. European Journal of Applied Physiology. 113: 127-134. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/22610152
24. Bin-Meferij MM, El-Kott AF. 2015. The radioprotective effects of Moringa oleifera against mobile phone electromagnetic radiation-induced infertility in rats. International Journal of Clinical and Experimental Medicine. 8: 12487-12497. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/26550159
25. Dawood MA, Koshio S, Ishikawa M, et al. 2016. Immune responses and stress resistance in red sea bream, Pagrus major, after oral administration of heat-killed Lactobacillus plantarum and vitamin C. Fish and Shellfish Immunology. 54: 266-275. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/27095173
26. National Research Council. Nutrient requirements of rabbits, 2nd revised edn. National Academy of Sciences, Washington, DC. USA. 1977.
27. Berkovich L, Earon G, Ron I, et al. 2013. Moringa Oleifera aqueous leaf extract down-regulates nuclear factor-kappaB and increases cytotoxic effect of chemotherapy in pancreatic cancer cells. BMC complementary and Alternative Medicine. 13: 212. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/23957955
28. Tuorkey MJ. 2016. Effects of Moringa oleifera aqueous leaf extract in alloxan induced diabetic mice. Interventional Medicine and Applied Science. 8: 109-117. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/28203392
29. Jang IS, Ko YH, Moon YS, et al. 2014. Effects of Vitamin C or E on the proinflammatory cytokines, heat shock protein 70 and antioxidant status in Broiler chicks under Summer conditions. Asian-Australasian Journal of Animal Science. 27: 749-756. Ref.: https://bit.ly/2QXIn4i
30. Deli? D, Gailus N, Vohr HW, et al. 2010. 6. Journal of Molecular Endocrinology. 45: 379-390.
31. Schltz LA. 1987. "Veterinary Haematology". 3rd ed., Lea and Febiger. 39: 217-222.
32. Costabile M. 2010. Measuring the 50% Haemolytic Complement (CH50) Activity of Serum. Journal of Visualized Experiments. 37: 1923. Ref.: https://bit.ly/2ulYDVa
33. Ohkawa H, Ohishi N, Yagi K. 1979. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry. 95: 351-358. Ref.: https://bit.ly/39OXIwE
34. Nishikimi M, Appaji N, Yagi K. 1972. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochemical and Biophysical Research Communication. 46: 849-854. Ref.: https://bit.ly/2tFHJ3n
35. Beutler E, Buron O, Kelly BM. 1963. Improved method for the determination of blood glutathione. Journal of Laboratory and Clinical Medicine. 6: 882-888.
36. Aebi H. 1984. Catalase in vitro. Methods Enzymol. 105: 121-126. Ref.: https://bit.ly/2T2PgEe
37. Berger A, Halver J. 1987. Effect of dietary protein, lipid and carbohydrate content on the growth, feed efficiency and carcass composition of striped bass, Morone saxatilis (Walbaum), fingerlings. Aquaculture Research. 18: 345-356. Ref.: https://bit.ly/36yppb7
38. Lee W, Ku SK, Na DH, et al. 2015. Anti-inflammatory effects of lysozyme against HMGB1 in human endothelial cells and in mice. Inflammation. 38: 1911-1924. Ref.: https://bit.ly/2QwNfyq
39. Stoyanchev K, Sotirov L, Bozakova N, et al. 2010. Natural humoral immunity in turkey breeders and broilers, healthy and with hereditary muscular dystrophy, reared under comfortable or stressful microclimatic conditions. Revue de Médicine Véterinaire. 161: 515-520. Ref.: https://bit.ly/39PwAh5
40. Panarelli M, Holloway CD, Mulatero P, et al. 1994. Inhibition of lysozyme synthesis by dexamethasone in human mononuclear leucocytes: an index of glucocorticoid sensitivity. Journal of Clinical Endocrinology and Metabolism. 78: 872-877. Ref.: https://bit.ly/2T2u8Om
41. Caruso D, Schlumberger O, Dahm C, et al. 2002. Plasma lysozyme levels in sheat fish Silurus glanis (L.) subjected to stress and experimental infection with Edwardsiella tarda. Aquaculture Research. 33: 999-1008. Ref.: https://bit.ly/36CX0Ru
42. Yousefi M, Paktinat M, Mahmoudi N, et al. 2016. Serum biochemical and non-specific immune responses of rainbow trout (Oncorhynchus mykiss) to dietary nucleotide and chronic stress. Fish Physiology and Biochemistry. 42: 1417-1425. Ref.: https://bit.ly/2N0KBis
43. Anjorin TS, Ikokoh P, Okolo S. 2010. Mineral composition of Moringa oleifera leaves, pods and seeds from two regions in Abuja, Nigeria. International Journal of Agriculture and Biology. 12: 431-434. Ref.: https://bit.ly/35wevl5
44. Ai Q, Mai K, Zhang C, et al. 2004. Effects of dietary vitamin C on growth and immune response of Japanese sea bass Lateolabrax japonicas. Aquaculture. 242: 489-500. Ref.: https://bit.ly/36ziaQq
45. Zhou C, Lin H, Huang Z, et al. 2014. Effect of dietary vitamin C on non-specific immunity and mRNA expression of three heat shock proteins (HSPs) in juvenile Megalobrama amblycephala under pH stress. Aquaculture. 434: 325-333. Ref.: https://bit.ly/2T75tYS
46. Pezzulo AA, Tang XX, Hoegger MJ, et al. 2012. Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature. 487: 109-113. Ref.: https://go.nature.com/2sVSfUb
47. Chen T, Cao X. 2010. Stress for maintaining memory: HSP70 as a mobile messenger for innate and adaptive immunity. European Journal of immunology. 40: 1541-1544. Ref.: https://bit.ly/35ut5JF
48. Takeda K, Kaisho T, Akira S. 2003. Toll-like receptors. Annual Reviews of Immunology. 2: 335-376. Ref.: https://bit.ly/39OyHBJ
49. Boshra H, Li J, Sunyer JO. 2006. Recent advances on the complement system of teleost fish. Fish and Shellfish Immunology. 20: 239-262. Ref.: https://bit.ly/2QwICEI
50. Rajanandh M, Kavitha J. 2010. Quantitative estimation of β-sitosterol, total phenolic and flavonoid compounds in the leaves of Moringa oleifera. International Journal of PharmTech Research. 2: 1409-1414. Ref.: https://bit.ly/2QQSW9m
51. Lin X. 2017. Quercetin protects against heat stroke-induced myocardial injury in male rats: antioxidative and antiinflammatory mechanisms. Chemico-Biological Interactions. 265: 47-54. Ref.: https://bit.ly/2QQRK61
52. Minaiyan M, Asghari G, Taheri D, et al. 2014. Anti-inflammatory effect of Moringa oleifera Lam. seeds on acetic acid-induced acute colitis in rats. Avicenna Journal of Phytomedicine. 4: 127-136. Ref.: https://bit.ly/2SZNcgf
53. Xu HJ, Jiang WD, Feng L, et al. 2016. Dietary vitamin C deficiency depressed the gill physical barriers and immune barriers referring to Nrf2, apoptosis, MLCK, NF-κB and TOR signaling in grass carp (Ctenopharyngodon idella) under infection of Flavobacterium columnare. Fish and shellfish immunology. 58: 177-192. Ref.: https://bit.ly/39Waium
54. Mandl J, Szarka A, Bánhegyi G. 2009. Vitamin C: update on physiology and pharmacology. British Journal of Pharmacology. 157: 1097-1110. Ref.: https://bit.ly/35BqmOR
55. Emeis M, Sonntag J, Willam C, et al. 1988. Acidosis activates complement system in vitro. Mediators of Inflammation. 7: 417-420. Ref.: https://bit.ly/2Qwvqzy
56. Kenawy HI, Boral I, Bevington A. 2015. Complement-coagulation cross-talk: a potential mediator of the physiological activation of complement by low pH. Frontiers in Immunology. 6: 215. Ref.: https://bit.ly/2N42GMr
57. Sotirov L, Koynarski T, Semerdjiev V, et al. 2011. Effect of breed upon blood lysozyme and complement activity in different sheep breeds. Journal of Agricultural Science and Technology. 3: 302-305. Ref.: https://bit.ly/39GuPD3
58. Letty J, Marchandeau S, Clobert J, et al. 2000. Improving translocation success: an experimental study of antistress treatment and release method for wild rabbits. Animal Conservation. 3: 211-219. Ref.: https://bit.ly/39NAWpd
59. Letty J, Marchadeau S, Reitz F, et al. 2002. Survival and movements of translocated wild rabbits (Oryctolagus cuniculus). Game & Wildlife Science. 19: 1-23. Ref.: https://bit.ly/35wA2K4
60. Lin H, Decuypere E, Buyse J. 2006. Acute heat stress induces oxidative stress in broiler chickens. Comparative Biochemistry and Physiology Part A: Molecular and Integrative Physiology. 144: 11-17. Ref.: https://bit.ly/2QveUzE
61. Khan RU, Naz S, Nikousefat Z, et al. 2012. Effect of ascorbic acid in heat-stressed poultry. World's Poultry Science Journal. 68: 477-490. Ref.: https://bit.ly/37Gg01m
62. Liu JQ, Manouchehri N, Lee TF, et al. 2012. Infusing sodium bicarbonate suppresses hydrogen peroxide accumulation and superoxide dismutase activity in hypoxic-reoxygenated newborn piglets. PloS one. 7: 39081. Ref.: https://bit.ly/2QQT7lb
63. Luqman S, Srivastava S, Kumar R, et al. 2012. Experimental assessment of Moringa oleifera leaf and fruit for its antistress, antioxidant, and scavenging potential using in vitro and in vivo assays. Evid-Based Complementary and Alternative Medicine. 2012: 1-12. Ref.: https://bit.ly/36xdMBd
64. Zhao B, Fei J, Chen Y, et al. 2014. Vitamin C treatment attenuates hemorrhagic shock related multi-organ injuries through the induction of heme oxygenase-1. BMC Complementary and Alternative Medicine. 14: 442. Ref.: https://bit.ly/2tBSGDc
65. Zhou Y, Yang W, Li Z, et al. 2018. Moringa oleifera stem extract protect skin keratinocytes against oxidative stress injury by enhancement of antioxidant defense systems and activation of PPARα. Biomed Pharmacotherapy. 107: 44-53. Ref.: https://bit.ly/2QueGci
66. Peng L, Zhang Z, Lan CQ, et al. 2017. Alleviation of oxygen stress on Neochloris oleoabundans: effects of bicarbonate and pH. Journal of Applied Phycology. 29: 143-152. Ref.: https://bit.ly/37FlZ6H
67. Guindon J, Hohmann AG. 2013. Use of sodium bicarbonate to promote weight gain, maintain body temperature, normalize renal functions and minimize mortality in rodents receiving the chemotherapeutic agent cisplatin. Neuroscience Letters. 544: 41-46. Ref.: https://bit.ly/2SXII9M
68. Voicule? C, Zar? O, Bogeanu C, et al. 2016. The role of oral sodium bicarbonate supplementation in maintaining acid-base balance and its influence on the cardiovascular system in chronic hemodialysis patients-results of a prospective study. Journal of Medicine and Life. 9: 449-454. Ref.: https://bit.ly/37K6uug
69. Zhu YB, Zhang YP, Zhang J, et al. 2016. Evaluation of vitamin C supplementation on kidney function and vascular reactivity following renal ischemic injury in mice. Kidney and Blood Pressure Research. 41: 460-470. Ref.: https://bit.ly/39ITXce
70. Abarikwu SO, Benjamin S, Ebah SG, et al. 2017. Protective effect of Moringa oleifera oil against HgCl2-induced hepato- and nephro-toxicity in rats. Journal of Basic and Clinical Physiology and Pharmacology. 28: 337-345. Ref.: https://bit.ly/2QtGRbg
71. Bailey M, McGuinness S, Haase M, et al. 2015. Sodium bicarbonate and renal function after cardiac surgery: a prospectively planned individual patient meta-analysis. Anesthesiology. 122: 294-306. Ref.: https://bit.ly/2ZWFEfy




















