Indexing & Abstracting
Full Text
Original ArticleDOI Number : 10.36811/jvsr.2021.110015Article Views : 86Article Downloads : 67
Effects of Vitamin C and Dimethylsulfoxide (Dmso) on Neuro-Behaviour, Apoptosis and oxidative stress in ischaemic stroke induced rats
Suleiman Nasiru1, Bulama Ibrahim2*, Lawal S Bilbis3, Saidu Yusuf3, Abdullahi Y Abbas3, Salisu Buhari4, Balarabe Salisu5 and Akib A Jimoh6
1Department of Veterinary Physiology and Biochemistry Usman DanFodiyo University Sokoto, Sokoto Nigeria
2Department of Veterinary Physiology and Biochemistry, University of Maiduguri, Maiduguri Nigeria
3Department of Biochemistry Usman Dan Fodiyo University Sokoto, Sokto Nigeria
4Department Veterinary Surgery and Radiology, Usmanu Danfodiyo University, Sokoto Nigeria
5Department of Medicine UsmanDanFodiyo University Teaching Hospital Sokoto, Sokot Nigeria
6Department of Theriogenology and Animal Production, Usmanu Danfodiyo University, Sokoto, Nigeria
*Corresponding Author: Bulama Ibrahim, Department of Veterinary Physiology and Biochemistry, University of Maiduguri, Maiduguri, Nigeria, Tel: +2347031879206; Email: bulams79@unimaid.edu.ng
Article Information
Aritcle Type: Original Article
Citation: Suleiman Nasiru, Bulama Ibrahim, Lawal S Bilbis, et al. 2021. Effects of Vitamin C and Dimethylsulfoxide (Dmso) on Neuro-Behaviour, Apoptosis and oxidative stress in ischaemic stroke induced rats. J Veterina Sci Res. 3: 35-53.
Copyright: This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Copyright © 2021; Suleiman Nasiru
Publication history:
Received date: 26 July, 2021Accepted date: 02 August, 2021
Published date: 05 August, 2021
Abstract
Background: Researches over the years have identified excitotoxicity, oxidative stress, inflammation and cell death as key contributors underlying progression of lesion in ischaemic stroke (IS). This study reports the effect of vitamin C and dimethylsulfoxide (DMSO) in the management of ischaemic stroke (IS) in Wistar rats.
Method: Forty rats were divided into four groups of ten rats each. Groups 1, 2 and 3 were induced with IS by middle cerebral artery occlusion (MCAO). Anxiety like behavior and locomotor activity assessments were carried out. Group 1was treated with vitamin C (67.5mg/kg) and group 2 with DMSO (67.5mg/kg) orally for two weeks. Group 4 was neither induced nor treated. Brain tissue homogenates (cerebral cortex) were assessed for the level of calcium binding protein (S100B), antioxidant enzymes [Superoxide dismutase (SOD), Catalae (CAT), Glutathion peroxidase (GPx)] gene expressions, BCL2, Bax and Caspase3.
Result: The results of this study showed that in the IS rats, there was significant (P<0.05) increase in anxiety and a significant (P<0.05) decrease in the locomotor activity. There was also significant (P<0.05) increase in S100B, caspase 3 and Bax expressions, while there was significant (P<0.05) decrease in BCL2. Treatment with vitamin C and DMSO resulted in significant (P<0.05) increase in the locomotor activity and significant (P<0.05) decrease in anxiety. There was significant (P<0.05) increase in the expression of SOD, CAT and GPx genes. Also, there was significant (P<0.05) decrease in S100B level, caspase-3 and Bax expressions, while there was significant (P<0.05) increase in BCL2. Conclusion: Conclusively, the results of this research highlighted the potentials for the use of vitamin C and DMSO in the management of IS.
Keywords: Stroke; Antioxidant; Locomotor; Neuro-Behavioural; Dimethylsulfoxide Apoptosis; Oxidative Stress
Background
The pathophysiology of stroke is intricate and comprises several processes, that includes; excitotoxicity, energy failure, oxidative stress, necrosis or apoptosis, inflammation, and disruption of the blood-brain barrier (BBB) [1]. The importance of oxidative damage is increasingly recognized as part of many neurodegenerative diseases processes. Decrease in enzymatic antioxidant activity and excessive production of free radicals are regarded as the major source of oxidative stress (OS) which leads to neuronal injury such as DNA damage, lipid peroxidation, protein oxidation and the subsequent cellular dysfunction, and death in the CNS. Reactive nitrogen species (RNS) and reactive oxygen species (ROS) are part of both necrotic and apoptotic mechanisms for the cell death [2]. Ischaemic stroke brain is recognized by upsurges in inflammatory responses, OS, disruption of calcium homeostasis and high levels of several S100B, a calcium binding protein [3]. S100B in the nervous system is mostly intense in glial cells. It has both trophic and possibly toxic effects on neuritis and neurons signifying that increased level of S100B plays a significant role in the evolution of disease [4].
It is postulated that high concentrations of S100B is a major factor in the pathogenesis of neurodegenerative processes such as IS, Alzheimer’s disease (AD) possibly through the mechanisms of OS [5]. One of the prominent and early sign of susceptible neurons in neurodegenerative diseases is OS. Exposure to oxidative stress prompts the buildup of intracellular reactive oxygen species (ROS) that lead to cell damage in the form of lipid, protein and DNA oxidations. Elevated levels of ROS are linked with high deposition of amyloid β and creation of senile plaques, a symbol of neurodegenerative brain disease [6]. If higher ROS go beyond the basal level of cellular protective mechanisms, it will result in oxidative damage and cell death. Thus, elements that can decrease oxidative stress are assumed to be likely drug candidates for the treatment or preventive therapy of neurodegenerative diseases [7]. ROS are constantly formed during physiological processes in normal brain tissue, but are regulated by endogenous antioxidant defense mechanisms. When cerebral ischaemic injury occurs, free radicals are highly produced and results in deactivation of detoxification mechanisms due to redox imbalance in the endogenous antioxidant system. After cerebral ischemia, increase in levels of ROS lead to OS and consequent neuronal injury [8] there by making free radical a convincing curative target, and antioxidant one of the possible option because of the mechanism by which it works: (i) inhibiting free radical production; (ii) scavenging free radicals or (iii) promoting free radical degradation [9]. Therefore antioxidant strategies can either target up-regulation of endogenous antioxidants (SOD, CAT, GPx) or delivery of exogenous antioxidants (vitamin C, vitamin E, uric acid etc). Low molecular weight antioxidant (LMWA) have been found to increase the serum activities of antioxidants enzyme (SOD, CAT and GPx), reduced the oxidative stress index (MDA) and mitigate some neurological derangements associated with IS as shown in our previous research [10].
The single existing treatment of acute ischaemic stroke approved by the Food and Drug Administration (FDA) is recanalization with tissue-type plasminogen activator (t-PA). Nevertheless, in the United States, after years of t-PA administration, the clinical result of this treatment is still poor, due to the susceptibility to complications and also the short treatment widow [11]. Exogenous antioxidants have been shown to boost the endogenous antioxidant defenses and curtail the deleterious effects of ROS thereby reducing oxidative stress which is one of the major players in the pathogenesis of ischaemic stroke [12]. From the view of therapeutic potential, oxidative stress scavenging effects, it is therefore important to ascertain the possible reason whether the increase in the antioxidant enzymes activities and the decrease in the oxidative stress index, was owed to up-regulation of the enzymes or reduction in ROS production. The purpose of this study was to evaluate the role of vitamin c and DMSO on neuro-behavior, apoptosis and oxidative stress in ischaemic stroke induced rats.
Methods
Chemicals and Reagents
Ascorbic acid (vit C) and Dimethylsulfoxide (DMSO), were acquired from Cayman® chemical company, Ann Arbor, USA. Ketamine hydrochloride, xylazine and chloroform were obtained from Rotexmedica®, Trittau, Germany. Calcium binding protein S100B assay kits was obtained from Fine Biotech® Co., Ltd, Wuhan, China, Rabbit polyclonal anti caspase -3 specific primary antibody, rabbit polyclonal anti Bax specific primary antibody, rabbit polyclonal anti Bcl2 specific primary antibody (Biorad Laboratories, USA), Polyclonal Goat anti-Rabbit-FITC conjugated secondary antibody (Cell Signaling, California), 4, 6-diamidino-2-phenylindole dihydrochloride (DAPI) (Cell Signaling, California), fluorescence microscopic (Leica Microsystems Limited, Heerbrugg, Switzerland).
Experimental Animals
Apparently, forty (40) healthy rats weighing between 250-300g of Wistar strain were acquired from the animal house. The rats were allowed to acclimatize to the research laboratory conditions and then divided into four groups of ten rats each. The rats were exposed to a 12 hours light/12 hour dark schedule. The rats were maintained on Rats Pellet (vital® feed), and clean water ad-libitum.
Experimental Design
The rats were shared into four groups of ten rats each. Groups I and II were induced with ischaemic stroke, group I was treated with vitamin C, while group II was treated with DMSO at the dose rate 67.5mg/kg body weight. Groups III was induced with ischaemic stroke but not treated (SINT) while group IV was used as negative control; non-stroke induced non-treated (NSINT). Vitamin C and DMSO were administered orally for two weeks. Comparison was done between group III and IV while group I and II were compared to group III. This work was approved by the animal care and utilization committee of Faculty of Veterinary Medicine. This study followed the principles of the Declaration of Helsinki. The experiment in this work does not involve human participant and therefore participation consent was not applied.
Ischaemic Stroke Induction
Middle Cerebral Artery Occlusion (MCAO) method as reported previously [13] was used with some modification. Xylazine and Ketamine were used to anaesthized the rats at the doses of 5mg/kg and 80mg/kg body weight respectively. The condition was sustained until the end of the occluding period. The hair in the neck region of the rats were shaved and scrubbed with savlon, to gain access to the main carotid artery (CCA), incision was made under sterile condition. The artery was ligated proximally, a nitch incision was created on the internal carotid artery distally using 25G needle, and an absorbable suture material was inserted through the hole into the artery until resistance felt. A silicon-coated suture material (coating diameter and length 0.35 and 5mm, respectively) was maneuvered through the internal and external carotid arteries to block the MCA. All the openings were closed using a non-absorbable closure material; nylon. The rats were then allowed to recover from anesthesia in the cages. During the surgery, the heart rate was monitored, and rectal temperature were regulated or maintained at 330-480 beats per minute and 35.9-37.5ºC respectively.
Neurological Assessments
Evaluation of Anxiety like behavior using Elevated plus Maze
Principle: The behavior of rat place on elevated position will be guided by exploration and fear, decrease exploration of the open arm will be used as an index of anxiety. The plus-maze consisted of two open (50x10 cm) and two enclosed (50x10x40 cm) arms, facing each other and elevated at a height of 50 cm. This apparatus was used to measure the anxiety of the IS rats. The rats were placed individually at the center and the time animal spent in each arm was noted. The animals were allowed to explore the apparatus for 5minutes. Animal tracking system was used to tracts and records the activity of each rat (ANY-maze 6.06 from Stoelting company, USA) [14].
Assesment of locomotor activity with Open Field Test
Principle: Rats with impaired muscle function are weak with low activity and have decrease total distance travelled while rats with unimpaired muscle function will be active and have increase total distance travelled. The open field apparatus was white plywood constructed and it was measured 100x100cm with 30 cm walls. One of the walls was clear Plexiglas, so that rat can be seen in the 2 apparatus. A computer-operated Digital camera system using ANY-maze software was used to recorded activities in the open-field. Total distance (locomotor activity) center distance (the distance traveled in the center of the arena) was recorded. The total distance was used as an index of locomotor activity [15].
Brain extraction and Homogenization
This was done according to standard procedure. The skin was opened at the midline of the head cutting from the roof of the skull to the mid-eye area using micro dissecting scissors. After folding back the skin flaps with the scissors, the skull was cut at the midline fissure, without cutting into the brain tissue. The raised skull cap was removed with the curved forceps, applying slight pressure. The brain was then released from the skull cavity by running a micro spatula underneath and along the length of the brain from the olfactory lobes to the beginning of the spinal cord. After gently transferring the brain to a 60 mm petri dish, the tissues were rinsed with a phosphate buffered saline (PBS) to remove any red blood cells and clots. Then the brains were transferred to a second petri dish and cut into small pieces in ice chilled10% PBS solution, slices were sonicated for 45 min in 100 cycle. Extract was separated from tissue by centrifugation at 1500 rpm for 5min, then supernatant was collected and use for the assay [16].
Biochemical Analyses
Marker of neuronal cell injury (S100B), antioxidant enzymes gene expressions and the expressions of apoptotic biomarkers (BCL2, Bax and Caspase-3) were assessed from the cerebral cortex.
Estimation of Calcium Binding Protein S100B
Principle: The assay was done using ELISA kit from Fine Biotech based on the principle of sandwich ELISA [17].
Gene expression study: SOD, CAT and GPx genes were assessed in this study in other to coroborate the effect of vitamin C and DMSO on the activities of antioxidant enzymes in IS rats.
Primer Design: Primer was designed using standard method [18]. The sequence obtained was used to order for the primers. Table 1 below shows the primer sequences and expected product size.
|
Table 1: Primers used and PCR product size. |
|||
|
Gene |
Forward primer |
Reverse primer |
Product Size |
|
SOD |
5`ACTTCGAGCAGAAGGCAAGC-3` |
3`TGAGGTCCTGCAGTGGTACA-5` |
133 bp |
|
GPx |
5`-GGACATCAGGAGAATGGCAAG-3` |
3`TCGATGTCGATGGTGCGAAA-5` |
323 bp |
|
CAT |
5`-GGTCTGGGACTTCTGGAGT-3` |
3`GATGGGTAATTGCCACTGG-5` |
285bp |
|
Beta actin |
5`- ACAACCTTCTTGCAGCTCCT-3` |
3`CCCATACCCACCATCACACC-5` |
185bp |
RNA Extraction
Procedure
The brain homogenate from all the IS rats were used for RNA extraction following the protocol described earlier [19]. A 250 ????L brain homogenate was dispensed in a 1.5mL sterile Eppendorf tubes and 750 ????l of Trizol LS was added in 1:3 ratio. The mixed sample was re-suspended by numerous pipetting in up and down manner and was later allowed to settle at room temperature for 15 minutes. About 200 ????Lof Chloroform was added to the mixture, and was vigorously shaken for 15 seconds, then allowed to settle for 5 minutes at room temperature before centrifugation at 12000 ×g for another 15minutes at 4?C. The clear aqueous phase at the upper part containing RNA was softly detached from the two organic and DNA phases into new 1.5mL labeled tube. Five hundred (500) microlitres of 100% isopropanol was added to each tube at room temperature for 10 minutes and allowed to stand before centrifugation at 12000 ×g for 10 minutes and the isopropanol was thrown away whereas the RNA was rinsed with alcohol (1000 ????L of 75%) and centrifuged for 5 minutes at 7500 ×g. The alcohol was discarded and the RNA pellet was partly desiccated inside level 2 biosafety cabinets for 5 to 10 minutes at the end of which 35 ????L of sterile RNAse free water was added to re-suspend the RNA for determination of purity, concentration, and later use for downstream application. The RNA purity and concentration was determined using NanoDrop Biospectrophotometer machine (NanoDrop1000 Thermoscientific).
Complimentary DNA (cDNA) Synthesis
Principle: The principle is based on the reverse transcription, where reverse transcriptase enzyme converts RNA to cDNA using oligo (dT) and random hexamer primers [20].
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) Amplification
Principle: Real-time PCR encompasses the primer mediated enzymatic amplification of DNA. PCR is used based on the ability of DNA polymerase to produce new strand of DNA complementary to the offered template strand as shown in table 1 Primer of interest is required because DNA polymerase can increase a nucleotide only onto a pre-existing 3′-OH group to add the first nucleotide. DNA polymerase then stretches its 3 ends by adding extra nucleotides to produce an extended region of double stranded DNA [21]. The cycling condition for PCR is presented in table 2 Agarose Gel Electrophoresis technique was also use to detect the targeted genes (SOD,CAT and GPx) [22].
|
Table 2: PCR Cycling Condition |
|||
|
Cycles |
Temperature(ºC) |
Time (s) |
Steps |
|
1 |
95 |
120 |
Initial denaturation |
|
40 |
95 |
5 |
Denaturation |
|
40 |
60 |
20 |
Annealing |
|
40 |
60 |
20 |
Extension |
Relative Gene Quantification
The threshold cycle (CT) values were measured to detect the threshold of each of three genes of interest and Beta actin gene in all samples based on standard method [23].
Immunoflourescence
Principle: Immunofluorescence is a method based on an antigen-antibody reaction where the antibodies are marked (labeled) with a fluorescence dye and the antigen-antibody complex is visualized using ultra-violet (fluorescent) microscope [24].
Procedure
The tissues were fixed with immune-stained and 10% neutral buffered formalin as follows; using ice cold PBST (PBS containing 0.5% Tween 20), the specimen were washed three times for 5 minutes. After this, the slides were incubated in a 0.5% Triton X-100 in PBST (PBS 1 L pH 7.2 + 0.5mL Tween 20) for 15 minutes to permeabilize the tissues followed by rinsing three times for 5 minutes with PBST. Unspecific binding was blocked using blocking buffer (5% BSA in PBST) for 1 hour at room temperature. The slides were rinsed three times with PBST for 5 minutes and then 40 ????l of either rabbit polyclonal anti caspase -3 specific primary antibody, rabbit polyclonal anti Bax specific primary antibody or rabbit polyclonal anti Bcl2 specific primary antibody (Biorad Laboratories, USA) diluted at 1:200 with sterile distilled water was dropped on the cells followed by incubation at 4?C in a dark humidified chamber overnight. The slides were washed thrice for 5 minutes with PBST and 40 ????L of polyclonal Goat anti-Rabbit-FITC conjugated secondary antibody (Cell Signaling, California) and diluted at 1:200 with sterile distilled water was dropped on each slide and incubated at room temperature for 1 hour in the dark. The slides were rinsed with PBST for 5 minutes three times and 20 ????l of 4, 6-diamidino-2-phenylindole dihydrochloride (DAPI) (Cell Signaling, California) was added to the slides and incubated for 10 minutes at room temperature and the plates were briefly rinsed with PBST. The cover slips were dried and placed on a labeled clean glass slides using a mountant (DPX) for fluorescence microscopic examination (Leica microsystem limited, Heerbrug, Switzerland) [25].
Data Analysis
Results were analyzed with the aid of statistical package - SPSS version 22. Results were expressed as means ± SD. Furthermore, data were analyzed by one-way analysis of variance (ANOVA). Where the F values were significant, the Tukey post-hoc test was used to compare groups. Gene expression fold changes by RT PCR considered significant at two-fold cut-off (P<0.05).
Results
Neuro-Behavioral Assessment
Anxiety level of IS Rats after Supplementation with VC and DMSO: Effect of ascorbic acid and DMSO on the level of anxiety of IS rats are presented in Figure 1. The result showed that SINT group spent more time in closed arm of the elevated plus maze apparatus indicating anxiety, while Vitamin C and DMSO treated groups spent more time in the open arm of the apparatus indicating absence or reduced anxiety.
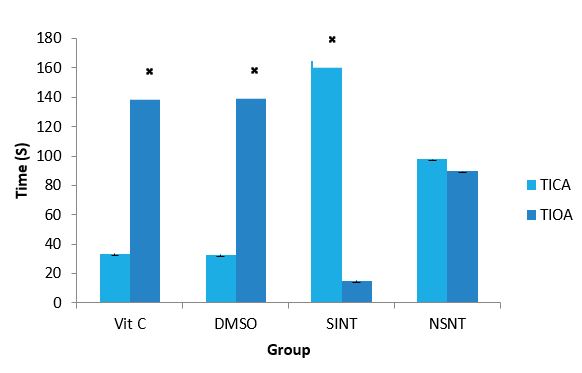
Figure 1: Effects of Vit C & DMSO on the level of anxiety of IS rats.
DMSO: Dimethylsulfoxide, Vit C: Vitamin C, SINT- Stroke induced non treated, NSNT- Non ischaemic stroke Non treated, TIOA- time in open arm, TICA- time in close arm. ? (P< 0.05) SINT versus Vit C and DMSO.
Locomotor activity of IS rats after supplementation with Vitamin C and DMSO: The effects of Vitamin C and DMSO on the locomotor activity of IS rats using open field test are presented in figure 2. SINT group demonstrated decreased locomotor activity (Decreased distance travelled) compared to the NSNT. However, both Vitamin C and DMSO treated groups did not exhibit motor defect as distance travelled is not significantly different from the NSNT group.
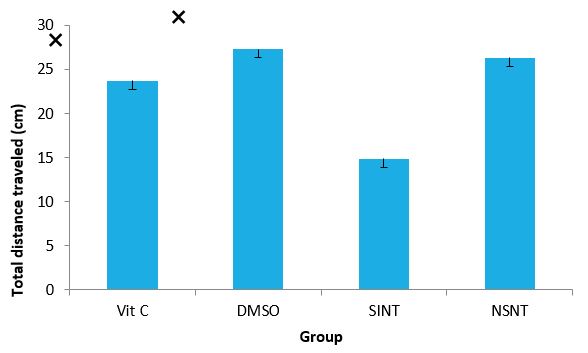
Figure 2: Effects of Vit C & DMSO on locomotor activity of IS rats.
DMSO: Dimethylsulfoxide, Vit C: Vitamin C, SIN: Stroke induced non treated, NSNT-Non ischaemicstroke Non treated ?(P< 0.05) SINT versus Vit C and DMSO.
Effect of Vitamin C and DMSO on the expression Level of CAT Gene of IS Rats: The effects of Vitamin C and DMSO on the expression of CAT gene of IS rats are presented in figure 3.Vitamin C and DMSO significantly (P<0.05) up-regulated CAT gene by 5.8 and 4.2 folds respectively.
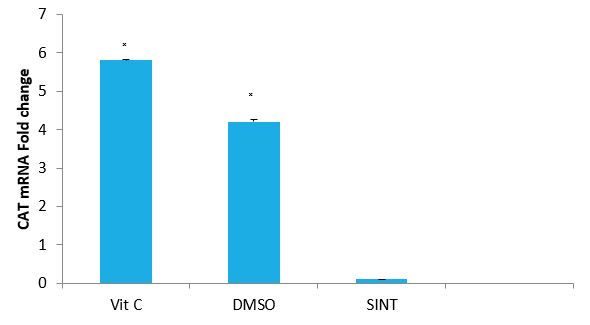
Figure 3: Effect of VitC and DMSO on the expression of CAT gene of IS rats by relative.
Quantification: Vit C: Vitamin C, DMSO: Dimethyl sulfoxide, SINT: Stroke induced non treated ?(P< 0.05) SINT versus Vit C and DMSO.
Effect of Vitamin C and DMSO on the expression Level of GPx Gene of IS Rats: The effects of Vitamin C and DMSO on the expression of GPx gene of IS rats are presented in figure 4.Vitamin C and DMSO treated groups, there was a significant (P<0.05) up-regulation of GPx gene by 6.5 and 4.1 fold changes respectively.
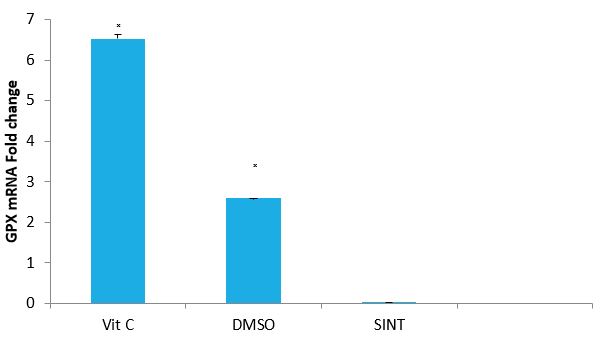
Figure 4: Effect of VitC and DMSO on the expression of GPX gene in IS rats by relative.
Quantification. Vit C: Vitamin C, DMSO: Dimethyl sulfoxide, SINT: Stroke induced non treated ?(P< 0.05) SINT versus Vit C and DMSO.
Effect of Vitamin C and DMSO on the expression Level of SOD Gene of IS Rats: The effects of Vitamin C and DMSO on the expression of GPx gene of IS rats are presented in figure 5.VitC and DMSO treated groups significantly (P<0.05) up-regulated SOD gene expression by 9.3 and 2.0 fold change respectively.
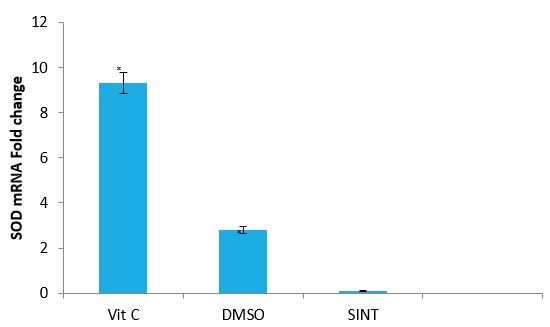
Figure 5: Effect of VitC and DMSO on the expression of SOD gene of IS rats by relative.
Quantification Vit C: Vitamin C, DMSO: Dimethyl sulfoxide, SINT: Stroke induced non treated ?(P< 0.05) SINT versus Vit C and DMSO.
Effect of Vitamin C and DMSO on the level of Calcium binding protein (S100B) of IS rats: The effects of Vitamin C and DMSO on the level of S100B of IS rats are presented in figure 6. The result of calcium binding protein (S100B) indicated that SINT group showeda significant (P<0.05) higher level of S100B compared to NSINT groupthat showed a significant (P<0.05) lower level. This indicates brain injury in the SINT rats. The results of the Vitamin C and DMSO treated groups showed significantly (P<0.05) lower level compared to the SINT group indicating reduction in injury.
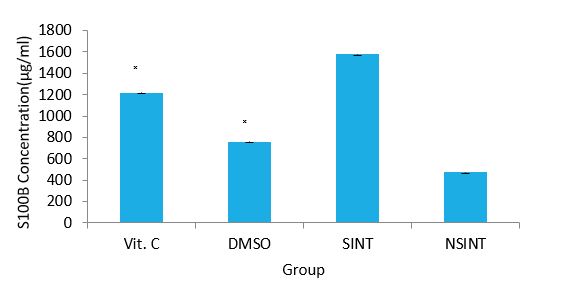
Figure 6: Effects of Vitamin C & DMSO on the level of S100B of IS rats.
DMSO: Dimethylsulfoxide, acid ,VC- Vitamin C, SINT- Stroke induced non treated, NSNT - Non ischaemic stroke Non treated ?(P< 0.05) SINT versus Vit C and DMSO.
The Effect of Supplementation of IS rats with Vitamin C and DMSO on the B-cell lymphoma (BCL2) protein expression: The effects of supplementation with Vitamin C and DMSO on an anti-apoptotic protein; B-cell lymphoma (BCL2) in IS rats are presented in plate 1. The results indicated a significant (P<0.05) increase in the expression of BCL2 in the treated groups while SINT group indicated a significant (P<0.05) decrease in the expression of BCL2.
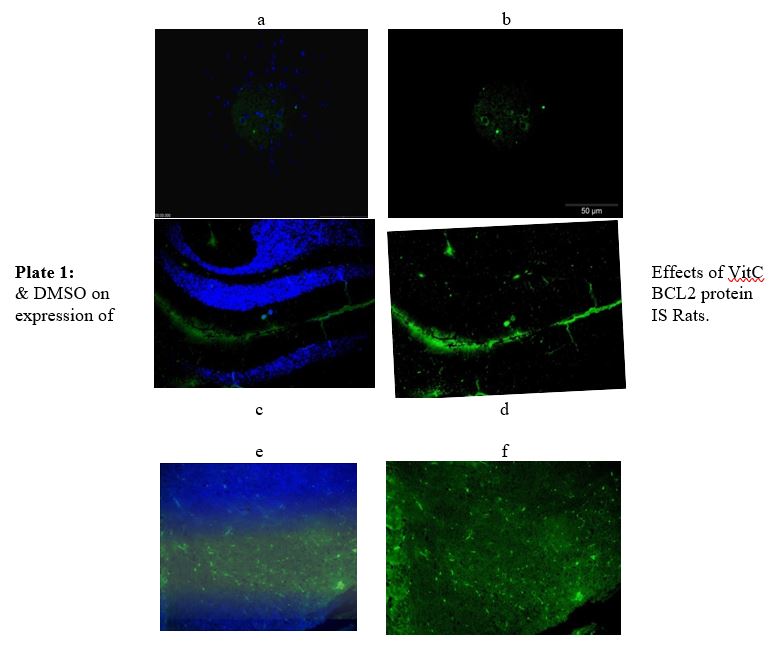
(Immunoflourescence based detectation) a- Merged (DAPI + FITC) from DMSO group, b- BCL2 (FIT C only) from DMSO group, c-Merged (DAPI + FITC) from SINT group, d- BCL2 (FIT C only) from SINT group, e - Merged (DAPI + FITC) from Vit C group, f- BCL2 (FIT C only) from VitC group, DMSO- Dimethylsulfoxide, Vit C- Vitamin C, SINT- Stroke induced non treated.
Effect of supplementation of IS rats with VitC and DMSO on Bax (BCL2 Associated X, Apoptosis Regulator) expression: The effects of VitC and DMSO supplementation on a pro-apoptotic protein (Bax) protein of IS rats are presented in plate 2. The results showed that there was an increase expression of Bax (a pro-apoptotic protein) in SINT group. Supplementation of DMSO and vitamin C however, indicated a decrease in the expression level of Bax.
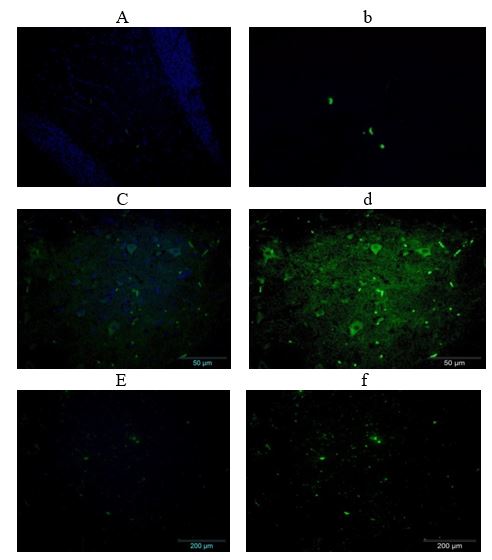
Plate 2: Effects of Vit C & DMSO on Bax expression of IS rats.
(Immunoflourescence based detection) a- Merged (DAPI + FITC) from DMSO group, b- Bax (FIT C only) from DMSO group, c-Merged (DAPI + FITC) from SINT group, d- Bax (FIT C only) from SINT group, e - Merged (DAPI + FITC) from VC group, f- Bax (FIT C only) from Vit C group, DMSO- Dimethylsulfoxide, Vit C- Vitamin C, SINT- Stroke induced non treated.
Effect of VitC and DMSO supplementation to IS rats on Caspase-3 expression: The effects of VitC and DMSO supplementation on a pro-apoptotic protein (caspase-3) of IS rats are presented in Plate 3. The results indicated that there was an increase expression of Caspase-3 in SINT group. Supplementation with VC and DMSO caused a decreased in the expression level of caspase-3.
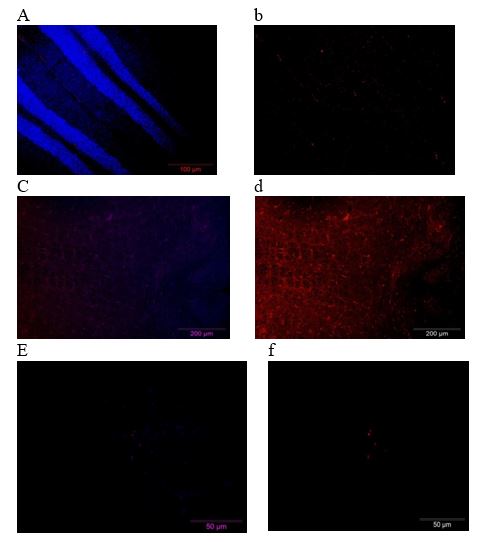
Plate 3: Effects of VitC & DMSO on Caspase-3 expression of IS rats. (Immunoflourescence based detection) a- Merged (DAPI + FITC) from DMSO group, b- Bax (FIT C only) from DMSO group, c-Merged (DAPI + FITC) from SINT group, d- Bax (FIT C only) from SINT group, e - Merged (DAPI + FITC) from Vit C group, f- Bax (FIT C only) from Vit C group, DMSO- Dimethylsulfoxide, Vit C- Vitamin C, SINT- Stroke induced non treated.
Discussion
Ischaemic stroke is triggered by a disruption of cerebral blood flow, typically through the middle cerebral artery (MCA), eventually leading to hypoperfusion of tissue and neuronal cell death [26]. Neurons are particularly susceptible to oxidative stress due to their high oxygen consumption and high metabolic activity that lead to high levels of ROS production, alongside relatively low levels of endogenous antioxidant enzymes, mainly the catalase [27]. It has been well recognized in epidemiological trials that, antioxidants reduce risk of stroke, as foods rich in vegetables and fruits help in managing modifiable risk factors for stroke incidence [28]. The results of this research on elevated plus maze test (TIOA and TICA) show that SINT group of the experimental rats spent more time in the close arm than the NSNT indicating anxiety which might be due to highly produced ROS that resulted into oxidative stress. There are new studies that propose a direct correlation between anxiety and oxidative stress. For instance, oxidative stress caused by L-buthionine- (S,R)-sulfoximine (BSO) in the amygdala and hypothalamus happens in parallel with anxiety-like behavioral patterns in mice [29]. In another study, on out bred Swiss mice, it was shown that increased anxiety-like behavior is positively correlated with rise in reactive oxygen species in granulocytes [30]. Meanwhile vitamin C and DMSO treatment groups spent more time in the open arm and less time in the close arm, indicating decrease in anxiety. Another report showed that supplementation of vitamin C lessened fear in male Japanese quail chicks [31]. In view of this finding, it will be expected that animals would spend more time on the open arms than in the closed arms (when elevated plus-maze test is carried out) owing to fearlessness. Nevertheless, in this current study, vitamin C has aided the entry of rats from the closed arms into the open arms, thereby demonstrating that fear factor is probably not involved.
The result of the antioxidant enzymes genes (SOD, CAT and GPx) of this study indicated that oxidative stress occurred following induction of IS. This is evident by the down regulation of SOD, CAT and GPx gene expressions observed in SINT group. Generally, it is well-known facts that SOD combat oxygen toxicity and catalytically convert the superoxide radical into the hydrogen peroxide which is disintegrated by CAT into oxygen and water. In that regard, oxidative damage caused by increase in the production of ROS after the induction of IS might have decrease the antioxidant defenses, producing protein oxidation and lipid peroxidation. Alteration in the balance between oxidation and reduction in a cell can alter the translocation of redox-sensitive transcription factors into the nucleus [32]. Down-regulation of CAT, SOD and GPx genes that was indicated in this study could be as a result of the oxidation of transcriptional factors that causes the initiation machinery of antioxidant enzymes transcription process. Vitamin C and DMSO supplementation resulted in significant (P<0.05) up-regulation of SOD, CAT and GPx genes, possibly, this could be due to transcriptional modification, because it was reported that [33], CAT activity is stimulated by phosphorylation at lower levels of ROS, while the activity is reduced at high levels of ROS by phosphatases that dephosphorylates the enzyme. Also, it is well documented in literature that redox regulation of transcription factors such as activator protein 1 (AP-1), nuclear factor kappa B and nuclear factor erythroid-derived 2-related factor 2 (Nrf-2) is significant in determining gene expression profile and cellular response to oxidative stress. The up-regulation recorded in this study following supplementation of vitamin C and DMSO could be ascribed to the ability of the antioxidants to be able to cause activation of transcriptional factors such as: AP-1 which could regulate cellular process like apoptosis, up-regulation of AP-1 could have activated the antioxidant gene expressions observed in this study. Vitamin C and DMSO may also have activated NF-kB which ultimately reduces inflammatory processes and apoptosis. Nrf-2 activation by the supplemented antioxidants might have enhances the levels of antioxidant enzymes and phase-2- detoxifying enzymes by complex mechanism [33].
The significant increase in the level of calcium binding protein S100Bin SINT group of IS rats is an indication that there is an injury, and this is supported by a previous report [34] where Neuron-specific enolase and S100B protein have been proposed as specific neuro-biochemical markers of brain injury following stroke, head trauma, and cardiac surgery. It was also reported that under normal conditions, concentration of neuron-specific enolase (NSE) and S100B are very little in the body system but when an ischaemic injury occur, they are released into the blood and the cerebrospinal fluid via the damaged BBB, leading to an increased level of S100B and NSE [34]. The Vitamin C and DMSO groups showed a significant decreased in the level of S100B respectively indicating the effect of vitamin C and DMSO treatments on IS rats. The increased level of S100B might have resulted from the increased ROS that would have caused OS. The treatment with vitamin C and DMSO were likely to have stopped the deleterious effect of ROS and OS, and thereby resulting in decreased S100B level. This is in line with the report that oxidative stress is related with the pathological process of the endothelial cell, including increasing permeability of the vascular endothelial cell, that affect cell proliferation, increasing infiltration of the leucocyte and interfering with the signal transduction in the cell, which causes apoptosis and cell death [36]. There was an increase in the expression of BCL2 which is an anti-apoptotic biomarkers in DMSO and Vitamin C treated group respectively as compared to SINT group where the expression was reduced or inhibited (plate 1), which could be an indication of apoptosis. It was reported that in stroke, apoptosis is a mechanism of deferred neuronal death in response to ischaemic injury. Main apoptotic proteins are activated and up-regulated after cerebral ischemia, whereas inhibition of these proteins prevents neurons from death [37]. Cascades are activated by regulated apoptotic pathways causing cell suicide without the leakage of cell contents that are harmful. Main actors in the control of apoptosis consist of proteins from the Bcl-2 family, Smac/DIABLO [38], [39] apoptotic protease-activating factor (Apaf-1) [40], [41] and HtrA2/Omi [42].Vitamin C in this study is able to reduce apoptosis by the increase in the expression of BCL2, this is in agreement with the findings that chronic ascorbic acid supplementation impedes apoptotic and pro-inflammatory changes and reduces brain injury and neurological deficits in STZ-induced diabetic rats [43].
Another study also reported that expression and transportation activity of sodium-dependent vitamin C transporter 2 after stroke are increased, which leads to a substantial increase of ascorbic acid uptake into the brain after stroke [44]. This could be the reason why vitamin C availability in this study led to the increased expression of an anti-apoptotic protein to neutralize the apoptosis as a result of ischaemic stroke. DMSO caused increased expression of BCL2 and it is in line with the work that reported the immunoblot analysis revealing a clear buildup of the anti-apoptotic BCL2 protein in DMSO-treated cells, compared to cells cultured for 96 h in medium without DMSO [45]. The BCL2 increase seemed to be explicitly caused by DMSO, as the protein level was unaffected in confluent compared to proliferating 3T3 cells. The report also recommended that, the prevention of high cell-density-dependent apoptosis in the presence of DMSO may be associated with an improved integrin-dependent cell-ECM adhesion and cadherin-mediated cell-cell contacts; furthermore, the consequence of DMSO on cell survival may be facilitated by increased levels of the anti-apoptotic BCL2 protein. This outcome was consistent with many earlier studies such as [46], where it was demonstrated that α5β1 integrin binding to fibronectin is significant to avert apoptosis in CHO cells upon serum withdrawal through BCL2 over expression. This study shows that Bax a pro-apoptotic biomarker increased significantly in SINT group indicating apoptosis (plate 2), which could have been initiated by oxidative stress. DMSO and vitamin C treated groups in the other hand resulted in the decreased level of Bax expression, and this could be traced to the ability of vitamin C to exert its antioxidant effect by reversing the oxidation. It has been demonstrated in a study which shows over stimulation of glutamate receptors induces abnormal calcium concentrations, which subsequently resulted into neuronal dysfunction and cell death or apoptosis [47]. Furthermore it was reported in a different study that in the developing fetal brain, two distinguished processes occur for cellular proliferation and differentiation, and the BCL2 family of proteins plays a crucial role in programmed cell death [48]. In their study, the levels of glutamate in the hippocampal segment of the developing brain was measured after administration of exogenous glutamate and vitamin C and also examined the response and changes in the expression of pro- and anti-apoptotic proteins. Though, the exact mechanism was not known to them, but their results clearly showed that vitamin C did not only decrease the levels of brain glutamate but also down-regulated the ratio of pro- to anti-apoptotic proteins in the hippocampal region of neonatal day 7 brains. It was observed that their results were in line with many previously reported in the literature where vitamin C reduced the glutamate-induced increase in Bax/BCL2 ratio in the rat thymus and exhibits a strong neuro-protective role [49].
Similarly, this study reported that caspase-3 significantly increased in SINT group indicating apoptosis (plate 3) and that could have been as a result of oxidative stress initiated by ROS. DMSO and vitamin C treated groups inhibits caspase-3 probably through scavenging ROS and amelioration of oxidative stress that is produced from stroke induction. The result of this study is consistent with the work which demonstrated that different doses of glutamate induced an increase in the Bax/BCL2 ratio which initiated the release of cytochrome c and the activation of caspase-3 [50]. The natural antioxidant vitamin C that was used as treatment in their research was capable of preventing cytochrome c translocation and the activation of caspase-3 against both doses of glutamate. As earlier reported, glutamate-dependent rises in calcium intracellular concentrations can persuade toxicity through oxidative stress by producing ROS, thus activating caspase-3 and cell death [51].
Conclusion
Ischemic stroke caused by the induction of middle cerebral artery occlusion in this study resulted in neuro-behavioral impairment, increased tendencies of apoptosis and oxidative stress. However, treatment with VC and DMSO separately resulted in significant improvement in the neuro-behavior of the IS rats, increase in the defense mechanism of the neuronal cell leading to prevention of apoptosis and reducing the tendency of oxidative stress. These are all evident by the investigative indices used in this study. Therefore, the use of VC and DMSO may be a hopeful strategy in the management of patients suffering from ischemic injury. The outcome of this work supported the neuroprotection and antioxidative strategy as a neurotherapeutic plan in stroke management.
List of Abbreviation
ATP: Adenosine Triphosphate; Vitamin E: Alpha tocopherol; AA: Arachidonic Acid; AD: Alzheimer’s Disease; ALA: Alpha Lipoic Acid; Vitamin C: Ascorbic acid; BBB: Blood Brain Barrier; BWT: Beam Walking Test; CAT: Catalase; CBF: Cerebral Blood Flow; CPP: Cerebral Perfusion Pressure; CRP: C-Reactive Protein; CSF: Cerebrospinal Fluid: CCA: Common Carotid Artery; CT Scan: Computed Tomography Scan; CT: Cylinder Test; CHD: Coronary Heart Disease; CNS: Central Nervous System; COX: Cyclooxygenase; DMSO: Dimethylsulfoxide; Dntp: Deoxyribonucleotide Triphosphate; ER: Endoplasmic Reticulum; GPx: Glutathione Peroxidase; H2O2: Hydrogen Peroxide; OGD: Oxygen and Glucose Deprivation; OH: Hydroxyl Radical; ICAM-1: Intercellular Adhesion Molecules-1; ICH: Intra Cerebral Hemorrhages; ICP: Intracranial Pressure; 1L-1: Interleukin-1; 1L-1β: Interleukin-1β; 1L-8: Interleukin-8; I/R: Ischemic Reperfusion; IS: Ischemic Stroke; LA: Lipoic Acid; LPO: Lipid Hydro Peroxide; LMWA: Low Molecular Weight Antioxidants; MCAO: Middle Cerebral Artery Occlusion; MCA: Middle Cerebral Artery; MAP Kinase: Mitogen-Activated Protein Kinases; MAO-B: Monoamine Oxidase B; MCP-1: Monocyte Adhesion Protein Chemistry -1; MDA: Malondialdehyde; MODS: Multiple Organ dysfunctions Syndrome; MRC: Mitochondrial Respiratory chain; NMDA: N-Methyl-D-Aspartic Acid; NMDAR: N-Methyl-D-Aspartic Acid Receptor; NO: Nitric Oxide; NOS: Nitric Oxide Synthase; NSNT: Non Stroke Induced Non Treated; PARP/AIF: Poly ADP-Ribose Polymerase/Apoptosis Inducing Factor; PCR: Polymerase Chain Reaction; PEG: Polyethylene Glycol; PNS: Peripheral Nervous System; ROS: Reactive Oxygen Species; rt-PA: Recombinant Tissue Plasminogen Activator; GSH: Reduced Glutathione; SCT: Stair Case Test; SEF: Secondary Energy Failure; SINT: Stroke Induced Non Treated; SOD: Superoxide Dismutase; TNF-α: Tumor Necrosis Factor; VCAM-1: Vascular Adhesion Molecules-1; WHO: World Health Organization; XDH: Xanthine Dehydrogenase; XO: Xanthine Oxidase.
References
1. Yiwang G, Pengyue L, Qingli G, et al. 2013. Pathophysiology and Biomarkers in Acute Ischaemic Stroke - A Review. Tropical Journal of Pharmaceutical Research.12: 1097-1105.
2. Valko M, Leibfritz D, Moncol J. 2017. Free radicals and antioxidants in normal physiological functions and human disease. International. Journal of Biochemistry and Cellular Biology. 39: 44-84. Ref.: https://pubmed.ncbi.nlm.nih.gov/16978905/ DOI: https://doi.org/10.1016/j.biocel.2006.07.001
3. Leclerc JL, Lampert AS, Diller MA. 2015. Genetic deletion of the pge2 ep3 receptor improves anatomical and functional outcomes after intracerebral hemorrhage. The European journal of neuroscience. 41: 1381. Ref.: https://pubmed.ncbi.nlm.nih.gov/25847406/ DOI: https://doi.org/10.1111/ejn.12909
4. Mark RE, Griffin WST. 2001. The role of activated astrocytes and of the neurotrophic cytokine S100B in the pathogenesis of Alzheimer’s disease. Neurobiology of Aging. 22: 915-922. Ref.: https://pubmed.ncbi.nlm.nih.gov/11754999/ DOI: https://doi.org/10.1016/s0197-4580(01)00293-7
5. Emanuele EV, Martinelli MV, Carlin E. 2011. Serum levels of soluble receptor for advanced glycation end products (sRAGE) in patients with different psychiatric disorders. Neuroscience letters. 487: 99-102. Ref.: https://pubmed.ncbi.nlm.nih.gov/20937362/ DOI: https://doi.org/10.1016/j.neulet.2010.10.003
6. Cheignona C, Tomasa M, Bonnefont-Rousselotc D. 2018. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biology. 14: 450-464. Ref.: https://pubmed.ncbi.nlm.nih.gov/29080524/ DOI: https://doi.org/10.1016/j.redox.2017.10.014
7. Nelson VM, Dancik W, Pan ZG. 2009. PAN-811 Inhibits Oxidative Stress-Induced Cell Death of Human Alzheimer's Disease-Derived and Age-Matched Olfactory Neuroepithelial Cells Via Suppression of Intracellular Reactive Oxygen Species. J of Alzheimer's Disease. 17: 611-619. Ref.: https://pubmed.ncbi.nlm.nih.gov/19433896/ DOI: https://doi.org/10.3233/jad-2009-1078
8. Facchinetti F, Dawson VL, Dawson TM. 1998. Free radicals as mediators of neuronal injury. Cell Mol Neurobiol. 18: 667-682. Ref.: https://pubmed.ncbi.nlm.nih.gov/9876873/ DOI: https://doi.org/10.1023/a:1020685903186
9. Margaill I, Plotkine M, Lerouet D. 2005. Antioxidant strategies in the treatment of stroke. Free Radic Biol Med. 39: 429-443. Ref.: https://pubmed.ncbi.nlm.nih.gov/16043015/ DOI: https://doi.org/10.1016/j.freeradbiomed.2005.05.003
10. Nasiru S, Bulama I, Abdurrahman JH. 2018. Neurobiochemical Roles of Low Molecular Weight Antioxidants on Oxidative Stress Biomarkers and Severity of Ischaemic Stroke in Wistar Rat. Journal of Neurology and Neurological Disorders. 4.
11. Saver JL. 2004. Number needed to treat estimates incorporating effects over the entire range of clinical outcomes - Novel derivation method and application to thrombolytic therapy for acute stroke. Archives of Neurology. 61: 1066-1070. Ref.: https://pubmed.ncbi.nlm.nih.gov/15262737/ DOI: https://doi.org/10.1001/archneur.61.7.1066
12. Ighodaro OM, Akinloye OA. 2018. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria Journal of Medicine. 54: 287-293.
13. Spratt NJ, Fernandez J, Chen M. 2006. Modification of the method of thread manufacture improves stroke induction rate and reduces mortality after thread-occlusion of the middle cerebral artery in young or aged rats. Journal of Neuroscience Methods. 155: 285-290. Ref.: https://pubmed.ncbi.nlm.nih.gov/16513179/ DOI: https://doi.org/10.1016/j.jneumeth.2006.01.020
14. Handley SL, Mithani S. 1984. Effects of Adreno-receptor Agonists and Antagonists in aMaze Exploration Model of Fear Motivated Behaviour. Naunyn Schmiedeberg's Archives of Pharmacology. 327: 1-5. Ref.: https://pubmed.ncbi.nlm.nih.gov/6149466/ DOI: https://doi.org/10.1007/bf00504983
15. Standford SC. 2007. The Open Field Test: reinventing the wheel. Journal of Psychopharmacology. 2007; 21: 134-135. Ref.: https://pubmed.ncbi.nlm.nih.gov/17329288/ DOI: https://doi.org/10.1177/0269881107073199
16. Rezanejad, K, Parivar K, Joghatayi M. 2014. Study of the differentiation of rat omentum stem cells to nerve cells using brain tissue extract of Wistar rats. International Journal of Cellular & Molecular. 2014: 1-13.
17. Ingebrigsten T, Romner B, Kongstad P. 1995. Increased Serum concentration of protein S100b after minor heat injury: a biochemical serum marker with prognostic value. Journal of Neurology, Neurosurgery and Psychiatry. 59: 103-104. Ref.: https://pubmed.ncbi.nlm.nih.gov/7608699/ DOI: https://doi.org/10.1136/jnnp.59.1.103-a
18. Rozen S. Skaletsky H. 2008. “Primer 3 on the WWW for general users and biologist programmers”In Krawetz S, Misener S. (eds. Pp365 386) Bioinformatc Methods and Protocols in series Metods in Molecular Biology, Humana Press. Totowa, NJ.
19. Chomczynski P, Mackey K. 1985. Short technical report: Modification of the TRIZOL reagent procedure for isolation of RNA from Polysaccharide-and proteoglycan-rich sources. Biotechniques. 19: 42-45. Ref.: https://pubmed.ncbi.nlm.nih.gov/8747660/
20. Skarratt KK, Fuller SJ. 2014. Quantitative Real-Time PCR Eliminates False-Positive in Colony Screening PCR. Journal of Microbiological Methods. 96: 99-100. Ref.: https://pubmed.ncbi.nlm.nih.gov/24291202/ DOI: https://doi.org/10.1016/j.mimet.2013.11.011
21. Mullis K, Faloona F, Scharf S. Specific enzymatic amplification of DNA in vitro: the polymerase chain reaction. Cold Spring HarbSymp. Quant Biol. 1986; 51: 263-273. Ref.: https://pubmed.ncbi.nlm.nih.gov/3472723/ DOI: https://doi.org/10.1101/sqb.1986.051.01.032
22. Johnson PH, Grossman LI. 1977. Electrophoresis of DNA in agarose gels. Optimizing separations of conformational isomers of double- and single-stranded DNAs. Biochemistry. 16: 4217-4225. Ref.: https://pubmed.ncbi.nlm.nih.gov/332225/ DOI: https://doi.org/10.1021/bi00638a014
23. Livak KJ, Schmittgen TD. 2001. Analysis of Relative Gene Expression Data Using Real-time Quantitative PCR and the 2-ΔΔCT.Method. 25: 402-408. Ref.: https://pubmed.ncbi.nlm.nih.gov/11846609/ DOI: https://doi.org/10.1006/meth.2001.1262
24. Mohan KH, Sathish P, Raghavendra R. 2008. Techniques of immunofluorescence and their significance. Indian J Dermatol Venereol Leprol. 74: 415-419. Ref.: https://pubmed.ncbi.nlm.nih.gov/18797086/ DOI: https://doi.org/10.4103/0378-6323.42898
25. Lawal N, Hair-Bejo M, Arshad SS. 2017. "Adaptation and Molecular Characterization of Two Malaysian Very Virulent Infectious Bursal Disease Virus Isolates Adapted in BGM-70 Cell Line", Advances in Virology, 2017:1-9. Ref.: https://pubmed.ncbi.nlm.nih.gov/29230245/ DOI: https://doi.org/10.1155/2017/8359047
26. Donnan RY, Moghaddas S, Hoppel CL. 2008. Ischaemic defects in the electron transport chain increase the production of reactive oxygen species from isolated rat heart mitochondria. Am J Physiol Cell Physiol. 294: 460-466. Ref.: https://pubmed.ncbi.nlm.nih.gov/18077608/ DOI: https://doi.org/10.1152/ajpcell.00211.2007
27. Dringen R. 2000. Metabolism and functions of glutathione in brain. Progress in Neurobiology. 62: 649-671. Ref.: https://pubmed.ncbi.nlm.nih.gov/10880854/ DOI: https://doi.org/10.1016/s0301-0082(99)00060-x
28. Joshipura KJ, Ascherio A, Manson JE. 1999. Fruit and vegetable intake in relation to risk of ischaemic stroke. Journal of American Medical Association. 282: 1233-1239. Ref.: https://pubmed.ncbi.nlm.nih.gov/10517425/ DOI: https://doi.org/10.1001/jama.282.13.1233
29. Masood A, Nadeem A, Mustafa SJ. 2008. Reversal of oxidative stress-induced anxiety by inhibition of phosphodiesterase-2 in mice. J Pharmacol Exp Ther. 326: 369-379. Ref.: https://pubmed.ncbi.nlm.nih.gov/18456873/ DOI: https://doi.org/10.1124/jpet.108.137208
30. Bouayed J, Rammal H, Younos C. 2007. Positive correlation between peripheral blood granulocyte oxidative status and level of anxiety in mice. Eur J Pharmacol. 564: 146-149. Ref.: https://pubmed.ncbi.nlm.nih.gov/17395178/ DOI: https://doi.org/10.1016/j.ejphar.2007.02.055
31. Jones RB, Satterlee DG, Cadd GG. 1999. Timidity in Japanese quail: effects of vitamin C and divergent selection for adrenocortical response. Physiol Behav. 67: 117-120. Ref.: https://pubmed.ncbi.nlm.nih.gov/10463637/ DOI: https://doi.org/10.1016/s0031-9384(99)00039-6
32. Sen CK, Packer L. 2000. Thiol homeostasis and supplements in physical exercise. Am J Clin Nutr. 72: 653-669. Ref.: https://pubmed.ncbi.nlm.nih.gov/10919972/ DOI: https://doi.org/10.1093/ajcn/72.2.653s
33. Cai YZ, Sun M, Corke H. 2003. Antioxidant activity of betalains from plants of the amaranthaceae. Journal of Agricultural and Food Chemistry. 51: 2288-2294. Ref.: https://pubmed.ncbi.nlm.nih.gov/12670172/ DOI: https://doi.org/10.1021/jf030045u
34. Herrmann M, Curio N, Jost S. 1999. Protein S-100B and neuron specific enolase as early neurobiochemical markers of the severity of traumatic brain injury. Restor Neurol Neurosci. 14: 109-114.
35. Van Munster BC, Korse CM, de Rooj SE. 2009. Markers of cerebral damage during delirium in elderly patients with hip fracture. BMC neurology. 9: 21. Ref.: https://pubmed.ncbi.nlm.nih.gov/19473521/ DOI: https://doi.org/10.1186/1471-2377-9-21
36. Ramagopal UA, Dulyanninova NG, Varney KM. 2013. Structure of the S100A4/myosin-IIA complex. BMC Struc Biol. 20: 13-31. Ref.: https://pubmed.ncbi.nlm.nih.gov/24252706/ DOI: https://doi.org/10.1186/1472-6807-13-31
37. Chen J, Nagayama T, Jin K. 1998. Induction of caspase-3-like protease may mediate delayed neuronal death in the hippocampus after transient cerebral ischemia. J Neurosci1. 8: 4914-4928. Ref.: https://pubmed.ncbi.nlm.nih.gov/9634557/ DOI: https://doi.org/10.1523/jneurosci.18-13-04914.1998
38. Du C, Fang M, Li Y. 2000. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 102: 33-42. Ref.: https://pubmed.ncbi.nlm.nih.gov/10929711/ DOI: https://doi.org/10.1016/s0092-8674(00)00008-8
39. Verhagen AM, Ekert PG, Pakusch M. 2000. Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell. 102: 43-53. Ref.: https://pubmed.ncbi.nlm.nih.gov/10929712/ DOI: https://doi.org/10.1016/s0092-8674(00)00009-x
40. Manfredi G, Beal MF. 2000. The role of mitochondria in the pathogenesis of neurodegenerative diseases. Brain Pathol. 10: 462-472. Ref.: https://pubmed.ncbi.nlm.nih.gov/10885665/ DOI: https://doi.org/10.1111/j.1750-3639.2000.tb00278.x
41. Yuan J, Yankner BA. 2000. Apoptosis in the nervous system. Nature. 407: 802-809. Ref.: https://pubmed.ncbi.nlm.nih.gov/11048732/ DOI: https://doi.org/10.1038/35037739
42. Suzuki Y, Imai Y, Nakayama H. 2001. A serine protease, HtrA2, is released from the mitochondria and interacts with XIAP, inducing cell death. Mol Cell. 8: 613-621. Ref.: https://pubmed.ncbi.nlm.nih.gov/11583623/ DOI: https://doi.org/10.1016/s1097-2765(01)00341-0
43. Iwata N, Okazaki M, Xuan M. 2014. Orally administrated ascorbic acid suppresses neuronal damage and modifies expression of SVCT2 and GLUT1 in the brain of diabetic rats with cerebral ischemia-reperfusion. Nutrients. 6: 1554-1577. Ref.: https://pubmed.ncbi.nlm.nih.gov/24739976/ DOI: https://doi.org/10.3390/nu6041554
44. Gess B, Sevimli S, Strecker JK. 2011. Sodium dependent vitamin C transporter 2 (SVCT2) expression and activity in brain capillary endothelial cells after transient ischemia in mice. PLoS ONE. 6: 17139. Ref.: https://pubmed.ncbi.nlm.nih.gov/21347255/ DOI: https://doi.org/10.1371/journal.pone.0017139
45. Mario F, Francesca D. 1999. Dimethyl Sulfoxide Restores Contact Inhibition-Induced Growth Arrest and Inhibits Cell Density-Dependent Apoptosis in Hamster Cells. Experimental Cell Research. 251: 102-110. Ref.: https://pubmed.ncbi.nlm.nih.gov/10438575/ DOI: https://doi.org/10.1006/excr.1999.4542
46. Zhang Z, Vuori K, Reed JC. 1995. The α5β1 integrin supports survival of cells on fibronectin and upregulates Bcl-2 expression. Proc Natl Acad Sci. 92: 6161-6165. Ref.: https://pubmed.ncbi.nlm.nih.gov/7541142/ DOI: https://doi.org/10.1073/pnas.92.13.6161
47. Sattler R, Tymianski M. Molecular mechanisms of calcium-dependent excitotoxicity. J Mol Med. 2000; 78: 3-13. Ref.: https://pubmed.ncbi.nlm.nih.gov/10759025/ DOI: https://doi.org/10.1007/s001090000077
48. Dunty JWC, Chen SY, Zucker RM. 2001. Selective vulnerability of embryonic cell populations to ethanol induced apoptosis: implications for alcohol-related birth defects and neurodevelopmental disorder. Alcohol ClinExp Res. 25: 1523-1535. Ref.: https://pubmed.ncbi.nlm.nih.gov/11696674/
49. Pavlovic V, Pavlovic D, Kocic G. 2009. Ascorbic acid modulates monosodium glutamate induced cytotoxicity in rat thymus. Bratislavske´ lekarskelisty. 110: 205-209. Ref.: https://pubmed.ncbi.nlm.nih.gov/19507646/
50. Shah SA, Yoon GH, Kim H. 2015. Vitamin C Neuroprotection against Dose?Dependent Glutamate?Induced Neurodegeneration in the Postnatal Brain. Neurochem Res. 40: 875-884. Ref.: https://pubmed.ncbi.nlm.nih.gov/25701025/ DOI: https://doi.org/10.1007/s11064-015-1540-2
51. Ekinci FJ, Linsley MD, Shea TB. 2000. Amyloid-induced calcium influx induces apoptosis in culture by oxidative stress rather than tau phosphorylation. Mol Brain Res. 76: 389-395. Ref.: https://pubmed.ncbi.nlm.nih.gov/10762716/ DOI: https://doi.org/10.1016/s0169-328x(00)00025-5




















