Indexing & Abstracting
Full Text
Case ReportDOI Number : 10.36811/ojor.2021.110010Article Views : 118Article Downloads : 85
Salivary Duct Carcinoma arising from pre-existing Epithelial-Myoepithelial Carcinoma of the parotid gland: Another rare example with Cytological Correlation
Hiroshi Harada1*, Mizue Sato2, Michiko Ehara3, Akihiko Kawahara4, Tetsuya Koyama5, Yusuke Miyamoto6, Seiya Kato2 and Akira Kurose1
1Department of Anatomic Pathology, Hirosaki University Graduate School of Medicine, Japan
2Division of Pathology, Saiseikai Fukuoka General Hospital, Japan
3Department of Oral Pathology, Oral Pathogenesis and Diseases Control, Asahi University School of Dentistry, Japan
4Department of Diagnostic Pathology, Kurume University Hospital, Japan
5Department of Otorhinolaryngology, Saiseikai Fukuoka General Hospital, Japan
6Department of Otorhinolaryngology and Head and Neck Surgery, Kyushu University Hospital, Japan
*Corresponding Author: Hiroshi Harada, DDS, PhD, Visiting Research Fellow, Department of Anatomic Pathology, Hirosaki University Graduate School of Medicine, 5 Zaifu, Hirosaki 036-8562, Japan, Tel: +81-172-39-5517; Fax: +81-172-39-5329; Email; harapppiii@kzd.biglobe.ne.jp
Article Information
Aritcle Type: Case Report
Citation: Hiroshi Harada, Mizue Sato, Michiko Ehara, et al. 2021. Salivary Duct Carcinoma arising from pre-existing Epithelial-Myoepithelial Carcinoma of the parotid gland: Another rare example with Cytological Correlation. Open J Otolaryngol Rhinol. 3: 01-12.
Copyright: This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Copyright © 2021; Hiroshi Harada
Publication history:
Received date: 25 January, 2021Accepted date: 03 February, 2021
Published date: 05 February, 2021
Abstract
An extremely exceptional case of salivary duct carcinoma (SDC) arising from pre-existing epithelial-myoepithelial carcinoma (EMC) of the parotid gland is herein described. The patient was a 50-year-old Japanese female, who presented with an asymptomatic mass of the right parotid gland that had been present for approximately 7 years. A superficial lobectomy of the parotid gland was performed and there have been no symptoms of recurrence or distant metastasis during over 10-year follow-up period. Histologically, the tumor was composed of an EMC component having double-layered ducts and invasive nests of myoepithelial clear cells with an SDC component intermingled throughout the lesion, which consisted of eosinophilic pleomorphic cells. Nests of the SDC component were occasionally accompanied by small luminal structures and minimally focalized comedo necrosis, and were immunoreactive for androgen receptor and GCDFP15. Fine needle aspiration cytology showed moderate cellularity, a few three-dimensional or sheet-like clusters, and single dispersed cells with abundant granular cytoplasm in the background. Furthermore, hyperchromatic small or flattened cells were identified attaching to the periphery of the clusters in retrospective observation. Metachromasia was also seen in May-Grunwald Giemsa stain.
Keywords: Salivary duct carcinoma; Epithelial-myoepithelial carcinoma; Parotid gland; Dedifferentiation; High-grade transformation; Fine needle aspiration cytology
Introduction
Salivary duct carcinoma (SDC) is histologically equivalent to invasive ductal carcinoma and is known as a high-grade carcinoma with an extremely poor prognosis among salivary gland tumors [1,2]. Apocrine-like characteristics are apparent in well-differentiated regions, and the expression of gross cystic disease fluid protein 15 (GCDFP15) and androgen receptor (AR) is generally regarded as a useful diagnostic index [3]. SDC usually occurs in the parotid gland of older males, but this is not the case with SDC arising ex pleomorphic adenoma (PA), which can occur in young adults, in females and in intraoral minor salivary glands.
On the other hand, epithelial-myoepithelial carcinoma (EMC) is a low- to intermediate-grade carcinoma comprising mainly double-layered ducts with inner luminal cells and outer myoepithelial clear cells in addition to clear cell nests. Various histological modifications such as apocrine-like or squamous metaplasia as well as sebaceous differentiation are sometimes observed [4,5], and the existence of cases with high-grade transformation (so-called dedifferentiation) is also known [6,7]. Herein, we describe an extremely rare case of SDC arising from pre-existing EMC of the parotid gland.
Case report
The patient was a 50-year-old Japanese female with a history of diabetes and hypertension, who presented with an asymptomatic mass of the right parotid gland that had been present for approximately seven years. The parotid mass had gradually increased in size, until she visited a local general hospital for surgical removal of the tumor. Magnetic resonance imaging (MRI) revealed a solid tumor measuring 13×10mm in greatest diameter, with a low signal on T1-weighted images and a slightly high signal on T2-weighted images (Figure1a). The clinical diagnosis was PA, and fine needle aspiration cytology (FNAC) was performed on the parotid gland tumor. The tumor was classified as indeterminate, favor low-grade malignant tumor by FNAC, and superficial lobectomy of the parotid gland was performed along with minor dissection of the regional lymph nodes. There have been no symptoms of recurrence or distant metastasis during over ten-year follow-up period.
Pathological findings
Gross and light-microscopic observation
On the cut surface of the gross specimen, the tumor presented a white to pale yellowish, well-circumscribed, but irregular-margined mass (Figure1b).

Figure 1: MRI reveals a solid tumor measuring 13×10mm in greatest diameter with a slightly high signal on T2W1 (a, arrow). On the cut surface of gross specimen, the tumor presents a white to pale yellowish, well-circumscribed, but irregular-margined mass (b).
Histologically, the tumor was composed of double-layered ducts consisting of inner luminal cells and outer myoepithelial clear cells mainly near the center of the lesion, and small invasive nests solely consisting of clear cells were sporadically found in the background of parotid parenchyma at the marginal area. There were also solid nests varying in size throughout the mass, which were consisted of pleomorphic cells having abundant eosinophilic granular cytoplasm and conspicuous nucleoli. Nests were occasionally accompanied by small luminal structures and minimally focalized comedo necrosis. Cord-like invasion was also noted in the fibrous stroma. Between these two components, there were transitional regions, where eosinophilic cells tended to form small, well-demarcated and smooth round-shaped nests, while the outer myoepithelial cells of double-layered ducts were occasionally compressed into a flattened shape by swollen inner luminal cells (Figure 2,3).
No lymph node metastasis was detected in any histological sections. Then, the tumor was classified as pT1N0M0, pStageI in accordance with the 8th Edition of Union for International Cancer Control (UICC) TNM classification of malignant tumors.
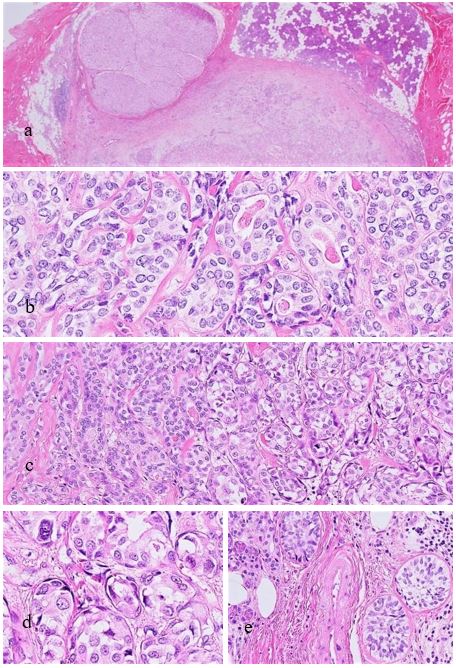
Figure 2: Light microscopy. The tumor presents a relatively well-defined but partially irregular and lobulated mass (a). The EMC component comprises of double-layered ducts composed of luminal cells in the inner layer and outer myoepithelial clear cells (b). In the transitional region, where both components meet, double-layered ducts have swollen inner luminal cells and small round or spindle-shaped cells derived from the outer myoepithelial cells of the EMC component are arranged at the periphery of the nests (c,d). Also, invasive clear cell nests are found in the marginal area (e).
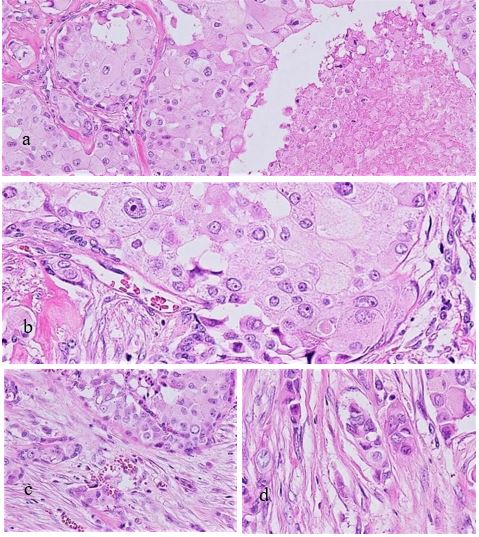
Figure 3: The SDC component is accompanied by comedo necrosis in large nests consisting of eosinophilic granular cells with irregular-shaped nuclei (a,b). The SDC component exhibits nesting or cord-like invasion into the fibrous stroma (c,d).
Immunohistochemistry
Eosinophilic granular cells were immunohistochemically positive for GCDFP15, AR and HER2 besides epithelial membrane antigen (EMA) and cytokeratin 7 (CK7), all of which were diffusely immunoreactive. Reactions for CK5/6 were generally limited, and myoepithelial markers such as S100 protein, vimentin, alpha-smooth muscle actin (aSMA), p63, and p40 were all negative. Conversely, myoepithelial cells of the double-layered ducts were positive for S100 protein, vimentin, aSMA, p63, p40 and WT1 (Wilms Tumor 1) protein (6F-H2). DOG1 was negative. Inner luminal cells were positive for EMA and CK7. Furthermore, AR and GCDFP15 were reactive corresponding to swollen cells in transitional regions. Thus, combined with light-microscopic features, a high-grade component consisting of eosinophilic pleomorphic cells was considered to be SDC. The other component mainly composed of double-layered ducts was determined as EMC. Ki-67 (MIB1) reacted more prominently in the SDC component than the EMC component (Figure 4,5).
It was presumed that the SDC component originated from the preceding EMC and that EMC caused invasive growth of solely the SDC component without myoepithelial cells into the fibrous stroma through cellular transformation of inner luminal cells.
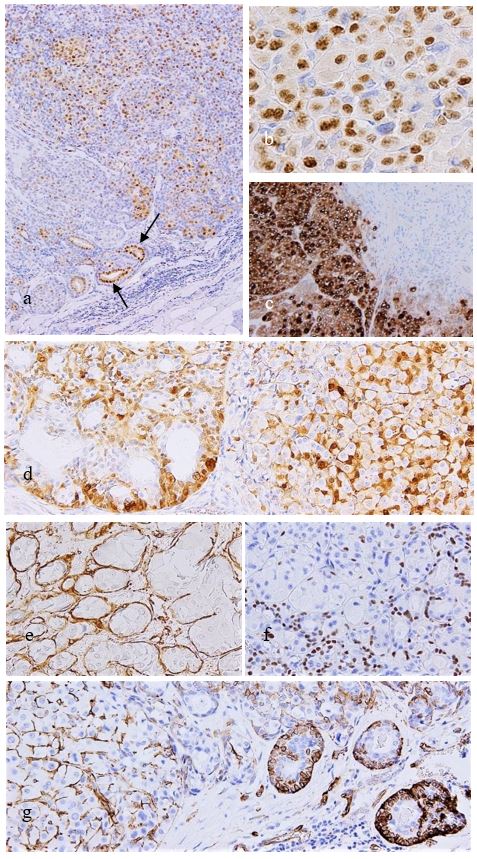
Figure 4: Immunohistochemistry. The SDC component is diffusely positive for AR (a,b), and some ducts of the EMC component are also positive for AR (arrows). Also GCDFP15 is diffusely positive (c) except the EMC component (right upper). S100 protein is widely positive and is generally consistent with the localization of other myoepithelial markers such as aSMA, p63 and also WT1 protein (d-g). Myoepithelial cell intervening between SDC tumor cells (d). Invasive ducts of EMC are seen in the marginal area (g).
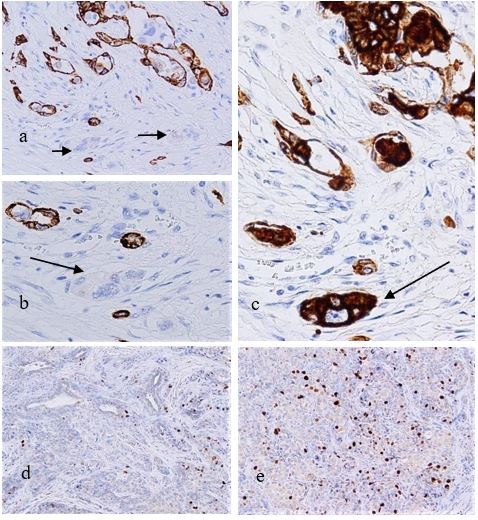
Figure 5: Immunohistochemistry. CK5/6 are positive corresponding to the outer myoepithelial cells of the EMC component, but invasive nests of SDC are not recognized (a,b), which are highlighted by CK7 (c). Arrows designating invasive nests. The SDC component shows a significantly higher Ki-67 labeling rate (maximum up to 13.4%) than the EMC component, and the number of positive cells in the EMC component is small (d,e).
Cytological findings
The aspirated material was 95% alcohol-fixed, and stained with Papanicolaou. Remaining specimens were dried and stained using the May-Grunwald Giemsa (MGG) method. The FNAC smear showed moderate cellularity, a few three-dimensional or sheet-like clusters, and single dispersed cells with abundant granular cytoplasm in the background. Although the nuclei were round to oval and of various sizes, with irregular and conspicuous nucleoli, the tumor cells with cytoplasmic vacuoles had a comparatively low nuclear/cytoplasmic (N/C) ratio with multinucleated cells occasionally intermingling. A few mitotic figures were observed. No background necrosis was observed as well as myxochondroid stroma reminiscent of PA. However, retrospective observation revealed metachromasia in MGG stain suggesting basement membrane-like substances or stromal mucin. In addition, in the nearby clusters, hyperchromatic small or flattened cells were attached to the periphery of the clusters, which were interpreted as remnants of outer myoepithelial cells derived from double-layered ducts of the EMC component, while eosinophilic granular cells were regarded as the SDC component (Figure 6).
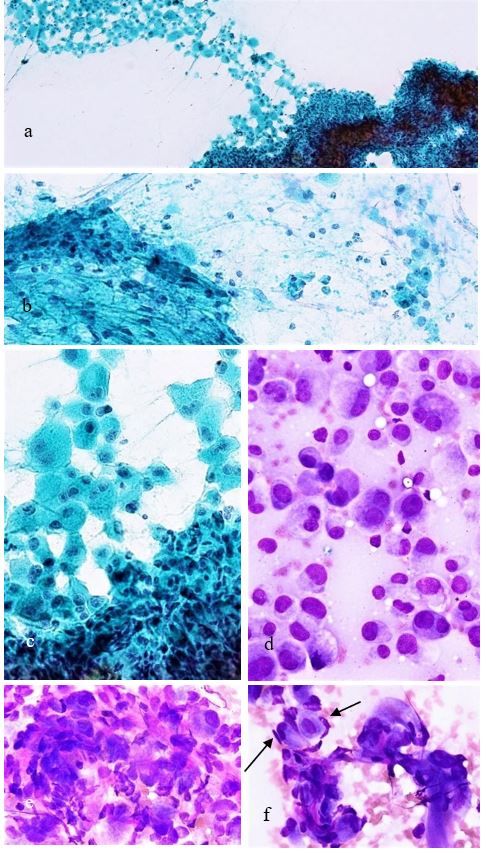
Figure 6: FNAC findings. The SDC component appears as a loosely bound or cohesive aggregates containing granular pleomorphic cells with prominent nucleoli (a-c). A few spindle-shaped cells are intermingled in a loose cluster (b). Pleomorphic cells are seen in also in MMG stain (d), occasionally associated with giant or multinuclear cells (e). Metachromasia is found within or around dense aggregates (e,f). Flattened myoepithelial cells of the EMC component are attached to the periphery of the aggregates (arrows). This finding reflects characteristics seen in Figure 2d.
Discussion
Histologically, the relationship between both components of SDC and EMC in the present case resembled that of SDC developing as carcinoma ex PA (CPA), but the characteristic localization of S100 protein was consistent with the characteristics of EMC and was not restricted to PA. EMC component was distinguishable from PA because double-layered ducts and the clear cell nests themselves showed overt invasive growth into the parotid parenchyma. Plasmacytoid cells and myxochondroid stroma characteristic of PA were completely lacking.
However, although the FNAC findings retrospectively well reflected the characteristics of the present tumor, no finding narrowed down the diagnosis to EMC simply on the basis of the myoepithelial cell aggregates, and ruling out CPA was virtually impossible, even if SDC could be detected and estimated and even if the general immunocytochemistry was applicable.
As mentioned above, EMC has apocrine-like features that are sometimes prominent, and Seethala et al. suggested that such a characteristic case could be a precursor lesion to hybrid tumor of EMC and SDC [4], although it should be termed as a hybrid carcinoma in this instance. In fact, a case remarkably similar to the present case was reported as a hybrid carcinoma of EMC and SDC in Japan [8]. Moreover, another rare case of SDC originating from an EMC was described in a report by Hamamoto et al. [9], in which one of us, the primary author (HH) participated. This case followed a lethal course due to diffuse invasive growth out of the parotid gland. Both cases can be regarded as actual cases proving the hypothesis by Seethala et al. [5], as well as our present case. Our case closely resembles the case reported by Hamamoto et al. with respect to the immunohistochemical finding except for the absence of DOG1 immunoreactivity, but differs regarding the lack of wide spread invasion and in following a favorable course, and thus is rather similar to the case by Kainuma et al. [8]. The proportion of both components was almost mutually equal in the present case. Furthermore, three cases of carcinoma arising ex basal cell adenoma have been described by Nagao et al. [10], among them, however, a case showing wide spread invasion and BRST1 immunoreactivity may require ruling out such cases described here, because EMC sometimes exhibits prominent basaloid features.
A hybrid tumor shows multiple different histological features that do not overlap with each other and is defined as a “neoplasm composed of two or more disparate patterns that are not included in each other histologic realm” [11]. However, in a narrow sense, while there is a description that limits the mutual relationship in terms of both differentiation directions and histogenesis [12], another study in which the target cases were collected without considering these viewpoints at all was reported [13]. Furthermore, clear references have not been made in the current WHO classification or AFIP Atlas of Tumor Pathology, and thus no established theory or consensus has been formed to date.
However, like hybrid carcinoma, dedifferentiated carcinoma is a carcinoma comprising a plurality of different histological appearances, and is characterized by the presence of a pre-existing low-grade carcinoma in addition to a dedifferentiated component with a high-grade malignant potential in histology. The dedifferentiated component generally takes the form of an undifferentiated carcinoma or high-grade adenocarcinoma NOS [14,15], but there is currently no description stating that the dedifferentiated component should not have distinct features that could be termed as a specific histological type such as that noted in hybrid carcinoma.
As stated similarly in the report by Hamamoto et al. [9], in the present example, the histological characteristics of the two components were clear and there was a marked difference between them. Thus, it seems that the interpretation of hybrid carcinoma is generally acceptable. However, because it contained an EMC component, as a low-grade carcinoma along with SDC component being a high-grade carcinoma, it could be interpreted as both a dedifferentiated carcinoma and a hybrid carcinoma. A narrower definition of hybrid tumors stated that "one has no evidence to be regarded as derived from the other" [11], but this has been removed from the revised second edition of this text for some reason. Apart from this conflicting background of the mechanism of occurrence, it would be currently and finally appropriate to treat also the present case here as a dedifferentiated carcinoma. Considering the general image of dedifferentiated carcinoma, including its "unknown" or “hard to name” high-grade component that leads to death, it is not unreasonable to presume that a certain number of pathologists will find this interpretation uncomfortable. However, the differences between the present case and that of Hamamoto et al. [9] may be merely the result of timing issue, and widespread invasion and death would ultimately occur, if the disease is left untreated for more years.
Conclusion
An extremely exceptional case of SDC arising from pre-existing EMC of the parotid gland was herein described. As described above, hybrid carcinomas and dedifferentiated carcinomas, which should be distinguished from each other in some cases as seemingly similar and different, are named and defined on the basis of their different characteristics or viewpoints. Therefore, there may be an inseparable, so to speak, histologically overlapping region between the two concepts, and the present case should be regarded as an example included in that region.
In conclusion, although extremely rare, such exceptional and complicated cases do exist and are virtually impossible to interpret strictly by cytology alone. But cytology could function successfully by guiding the next step, adequate surgical procedure.
Acknowledgment
The authors thank to Mr. Shinsuke Sato, CT, Division of Pathology, Saiseikai Fukuoka General Hospital for his revealing advice regarding cytological examination.
Note: In addition to being a research fellow at Hirosaki University Graduate School of Medicine, the primary author (HH) is also a visiting staff pathologist and a research fellow at Osaka International Cancer Institute.
References
1. Delgado R, Vuitch F, Albores-Saavedra J. 1993. Salivary duct carcinoma. Cancer. 72: 1503-1512. Ref.: https://pubmed.ncbi.nlm.nih.gov/7688652/ Doi: https://doi.org/10.1002/1097-0142(19930901)72:5%3C1503::aid-cncr2820720503%3E3.0.co;2-k
2. Barnes L, Rao U, Krause J, et al. 1994. Salivary duct carcinoma. Part I. A clinicopathologic evaluation and DNA image analysis of 13 cases with review of the literature. Oral Surg Oral Med Oral Pathol. 78: 64-73. Ref.: https://pubmed.ncbi.nlm.nih.gov/8078666/ Doi: https://doi.org/10.1016/0030-4220(94)90119-8
3. Fan CY, Wang J, Barnes EL. 2000. Expression of androgen receptor and prostatic specific markers in salivary duct carcinoma: an immunohistochemical analysis of 13 cases and review of the literature. Am J Surg Pathol. 24: 579-586. Ref.: https://pubmed.ncbi.nlm.nih.gov/10757407/ Doi: https://doi.org/10.1097/00000478-200004000-00014
4. Seethala RR, Barnes EL, Hunt JL. 2007. Epithelial-myoepithelial carcinoma: a review of the clinicopathologic spectrum and immunophenotypic characteristics in 61 tumors of the salivary glands and upper aerodigestive tract. Am J Surg Pathol. 31: 44-57. Ref.: https://pubmed.ncbi.nlm.nih.gov/17197918/ Doi: https://doi.org/10.1097/01.pas.0000213314.74423.d8
5. Seethala RR, Richmond JA, Hoschar AP, et al. 2009. New variants of epithelial-myoepithelial carcinoma: oncocytic-sebaceous and apocrine. Arch Pathol Lab Med. 133: 950-959. Ref.: https://pubmed.ncbi.nlm.nih.gov/19492889/ Doi: https://doi.org/10.1043/1543-2165-133.6.950
6. Kusafuka K, Takizawa Y, Ueno T, et al. 2008. Dedifferentiated epithelial-myoepithelial carcinoma of the parotid gland: a rare case report of immunohistochemical analysis and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 106: 85-91. Ref.: https://pubmed.ncbi.nlm.nih.gov/18417380/ Doi: https://doi.org/10.1016/j.tripleo.2008.01.013
7. Roy P, Bullock MJ, Perez-Ordonez B, et al. 2010. Epithelial-myoepithelial carcinoma with high grade transformation. Am J Surg Pathol. 34: 1258-1265. Ref.: https://pubmed.ncbi.nlm.nih.gov/20679885/ Doi: https://doi.org/10.1097/pas.0b013e3181e366d2
8. Kainuma K, Oshima A, Suzuki H, et al. 2010. Hybrid carcinoma of the parotid gland: report of a case (epithelial-myoepithelial carcinoma and salivary duct carcinoma) and review of the literature. Acta Otolaryngol. 130: 185-189. Ref.: https://pubmed.ncbi.nlm.nih.gov/19449226/ Doi: https://doi.org/10.3109/00016480902930458
9. Hamamoto Y, Harada H, Suzuki M, et al. 2020. Salivary duct carcinoma of the parotid gland originating from an epithelial-myoepithelial carcinoma: report of a rare case. Head Neck Pathol. 14: 283-289. Ref.: https://pubmed.ncbi.nlm.nih.gov/30937832/ Doi: https://doi.org/10.1007/s12105-019-01034-0
10. Nagao T, Sugano I, Ishida Y, et al. 1997. Carcinoma in basal cell adenoma of the parotid gland. Pathol Res Pract. 193: 171-178. Ref.: https://pubmed.ncbi.nlm.nih.gov/9198102/ Doi: https://doi.org/10.1016/s0344-0338(97)80074-x
11. Gnepp DR, Brandwein MS, Henley JD. 2000. Salivary and lacrimal glands, in “Diagnostic Surgical Pathology of the Head and Neck” In: Gnepp DR (ed). WB Saunders Co. Philadelphia, 325-430.
12. Grenko RT, Abendroth CS, Davis AT, et al. 1998. Hybrid tumors or salivary gland tumors sharing common differentiation pathways? Reexamining adenoid cystic and epithelial-myoepithelial carcinomas. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 86: 188-195. Ref.: https://pubmed.ncbi.nlm.nih.gov/9720095/ Doi: https://doi.org/10.1016/s1079-2104(98)90124-x
13. Nagao T, Sugano I, Ishida Y, et al. 2002. Hybrid carcinomas of the salivary glands: report of nine cases with a clinicopathologic immunohistochemical, and p53 gene alteration analysis. Mod Pathol. 15: 724-733. Ref.: https://pubmed.ncbi.nlm.nih.gov/12118110/ Doi: https://doi.org/10.1097/01.mp.0000018977.18942.fd
14. Stanley RJ, Weiland LH, Olsen KD, et al. 1988. Dedifferentiated acinic cell (acinous) carcinoma of the parotid gland. Otolaryngol Head Neck Surg. 98: 155-161. Ref.: https://pubmed.ncbi.nlm.nih.gov/3128758/ Doi: https://doi.org/10.1177/019459988809800210
15. Cheuk W, Chan JK, Ngan RK. 1999. Dedifferentiation in adenoid cystic carcinoma of salivary gland: an uncommon complication associated with an accelerated clinical course. Am J Surg Pathol. 23: 465-472. Ref.: https://pubmed.ncbi.nlm.nih.gov/10199477/ Doi: https://doi.org/10.1097/00000478-199904000-00012




















