Indexing & Abstracting
Full Text
Research ArticleDOI Number : 10.36811/ojprm.2019.110004Article Views : 40Article Downloads : 36
Effects of 1,8-cineole (eucalyptol) on the activity of human bronchial tissue
André-Michael Beer1*, Plamen Sagorchev2 and Julian Lukanov2
1Ruhr University Bochum, Im Vogelsang 5-11, 45527 Hattingen, Germany
2Medical University, Biophysics Department, 15-A V. Aprilov Street, 4000 Plovdiv, Bulgaria
*Corresponding Author: Prof. Dr. med. André-Michael Beer, MSc Ruhr-University Bochum, Im Vogelsang 5-11, 45527 Hattingen, Germany, Phone: 0049 2324 39672487; Fax: 0049 2324 39672497; Email: andre.beer@klinikum-Bochum.de
Article Information
Aritcle Type: Research Article
Citation: André-Michael Beer, Plamen Sagorchev, Julian Lukanov. 2019. Effects of 1,8-cineole (eucalyptol) on the activity of human bronchial tissue. Open J Pulm Respir Med. 1: 12-22.
Copyright: This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Copyright © 2019; André-Michael Beer
Publication history:
Received date: 12 November, 2019Accepted date: 22 November, 2019
Published date: 25 November, 2019
Abstract
1,8-cineole (eucalyptol) is used for the treatment of bronchial complaints, sinusitis and colds. Experiments have previously shown that 1,8-cineole, a monoterpene (C-10), has a very pronounced spasmolytic effect on smooth muscle fibre. In nearly all clinical applications with 1,8-cineole, especially when used in conjunction with allergic symptoms, histamine receptors prove to be crucial for treatment (use of histamine inhibitors).
Results: The desired 1,8-cineole effects are attained through:
- specifically blocking H1 histamine receptors, without influencing ACh receptors
- Inhibiting the contractile activity of human bronchial smooth muscle by activating H2 histamine receptors.
The ultimate goal of this study was to address the bronchodilatory effect of this compound, using human airway smooth muscle in order to demonstrate a possible role for 1,8-cineole in airway diseases.
Keywords: Smooth muscle fibre; 1,8-cineole (eucalyptol); Human bronchial tissue; Bronchitis; Monoterpene
Introduction
For many years, medications containing 1,8-cineole (eucalyptol) have been used for the treatment of bronchial complaints, sinusitis and colds. It has been previously shown that 1,8-cineole, a monoterpene (C-10) [1], has a very pronounced spasmolytic effect on smooth muscle fibres (SMF), similar to that of papaverine [2]. 1,8-cineole was found to have agonistic effects on the 1 and 2 adrenergic receptors [3]. These effects can be registered at low concentrations of up to 3 x10-7 M to 2 x 10-5 M 1,8-cineole. At higher concentrations, the well-known spasmolytic effect appears. At concentrations above 4 x 10-4 M 1,8-cineole, the effect of 10-5 M acetylcholine is 100% suppressed. The results of the interactions between 1,8-cineole and histamine indicate that 1,8-cineole is a reversible antagonist for histamine receptors. Increasing the “concentration” of 1,8-cineole by 16-fold (from 0.005 up to 0.080 l) increases this inhibition from approx. 25% to approx. 66% [4]. However, the various smooth muscles in the body are characterised by different types and number of receptors. The aim of this manuscript is to describe the effect of 1,8-cineole on the contractile activity of human bronchial smooth muscles.
Materials and Methods
In in vitro experiments on smooth muscle fibre of bronchial muscle, the influence of histamine under normal conditions and using different concentrations of 1,8-cineole on the spontaneous contractile activity (SCA) of SMF was investigated.
2.1 Measurement of the spontaneous contractile activity of smooth muscle fibre of segmental bronchi and method of preparation.
The measurements were performed according to the standardised Golenhofen method [5]. The smooth muscle fibre used in the experiments was taken from human segmental bronchi.
The muscle tissue had a length of 12-15 mm and a width of 1.5-2 mm and was taken from human segmental bronchi. Organ baths (20 mL volume) contained a Krebs´ solution (KS) with the following composition: mmol/L: Na+ (143), K+ (5.94), Mg2+ (1.19); Ca2+ (2.5), Cl- (133), HCO- (16.7), HPO42- (11.9) and glucose (11.5). A pH-value within physiological limits of 7.2 to 7.3 (7.2 ± 0.8) was maintained. The organ baths of Krebs´ solution were kept at 35 °C (± 0.2°C). Throughout the experiments, the Krebs´ solution was carbonated with carbon dioxide gas. The response to histamine was measured under isometric conditions (mN).
Substances
-Acetylcholine chloride (acetylcholine ophtalmicum dispersa®), Dispersa, Germering, Germany)
- Substances for Krebs´ solution (Merck comp., Darmstadt, Germany): NaCl, KCl, MgCl2 6 H2O, KH2PO4, NaHCO3, CaCl2
- 1,8-cineole, Ph. Eur., Ch.-B: 1,015,309 (purity 99.6%; Klosterfrau Healthcare Group, Berlin).
The following agonists and blockers were used in the assays:
• 1,8-cineole, Ph.Eur., Ch.-B:10153-09, Klosterfrau Healthcare Group, Berlin, Germany
• H1 agonist (2 - (2-pyridyl) - ethylamine), Sigma-Aldrich Co, USA
• α1/α2 antagonist (benextramine), RBISIGMA, St. Louis, MO, USA
• H2 agonist (dimaprit), Sigma-Aldrich Chemie GmbH, Germany
• H2 antagonist (famotidine), Sigma-Aldrich Chemie GmbH, Germany
• α2 agonist (methoxamine), RBISIGMA, St. Louis, MO, USA
• α1 agonist (p-iodoclonidine), RBISIGMA, St. Louis, MO, USA
Substances from Merck (Merck, Darmstadt, Germany) were used to prepare the prep and Krebs´ solution.
Cineole solution
At room temperature, 10 μL 1,8-cineole were added to 2 mL of ethanol (98%). The sample was homogenised with a vortexer for 2 min. The transparency of the samples was determined using standardised methodology at an illumination of 1500 lux. After proper solubility was established, 1,8-cineole was progressively diluted to 100 L 1,8-cineole in 2 mL of ethanol (98%) (5% solution of cineole in 98% ethanol). Further dilution with Krebs´ solution was performed. Thereafter, direct comparisons of the absorption spectra of the concentrations of 1,8-cineole in Krebs´ solution were determined.
Statistics
To take into account the specific variations and alterations of the SCA preparation’s measured values, the concentration-response curves in each case of N=10 individual experiments, always measured the excitation in % of maximal contractile activity of smooth muscle tissue when exposed to 10-5 M acetylcholine (ACh). The changes of the smooth muscle fibre (SMF) concerning SCA with various substances are given in Newtons (N). The processing of experimental data was performed using the Statistica 4.5 (StatSoft, Inc. Microsoft, USA) program. For comparison between two groups, the t-Test (student) for unpaired samples was implemented. For comparisons between three or more groups, variance analysis (ANOVA) was used. The statistical comparisons were performed at the 5% significance level. The results are expressed as mean±standard deviation. In each case n=7 measurements per experiment were performed.
Results
To analyse the effects and mechanisms of effect of 1,8-cineole on the contractile activity of the segmental bronchi, the tissue was first tested for the presence of α1, α2 adreno-, H1, H2 histamine and ACh receptors.
Figure 1: shows that 10-5 M ACh brings about very good contraction of the smooth muscle fibre of the segmental bronchi. It corresponds to the maximal attainable contractile effect of smooth muscles.
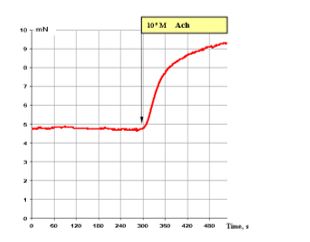
Figure 1: Effect of 10-5 M ACh on the contractile activity of smooth muscle fibre of human segmental bronchi.
Figure 2: (top) shows that 10-7 M p-iodoclonidine (α2 adrenoreceptor agonist) has a stimulating effect on these muscle preparations.
Figure 2: (bottom) shows that also 10-6 M methoxamine (α2-agonist) induces markedly stimulating effects.
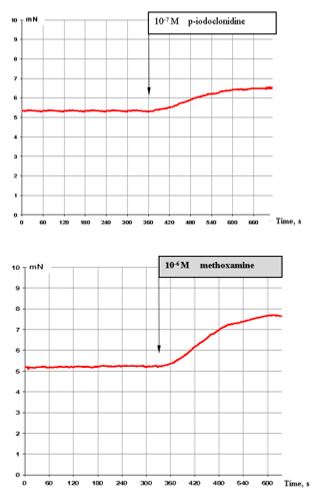
Figure 2: Effect of 10-7 M p-iodoclonidine (top) and 10-6 M methoxamine (bottom) on the contractile activity of smooth muscle fibre of human segmental bronchi.
Figure 3: illustrates the effects of H1 (bottom) and H2 (top) histamine agonists. Dimaprit, a H2 histamine receptor agonist, is a strong inhibitor of the contractile activity of segmental bronchi (Figure. 3, top).
By contrast, activation of H1 histamine receptors results in observation of highly stimulating effects, almost comparable with those of 10-5 M ACh (Figure. 3, bottom).
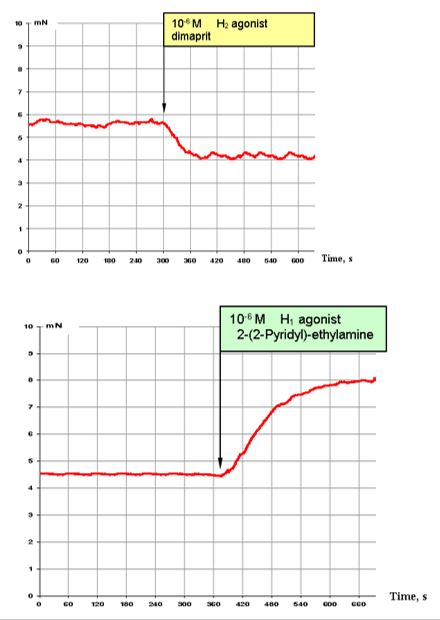
Figure 3: Effect of 10-6 M H2 agonist dimaprit (top) and effect of 10-6 M H1 agonist 2-(-2 -pyridyl)-ethylamine (bottom) on the contractile activity of smooth muscle fibre of human segmental bronchi.
Figure 4: (top) shows that, at an effect with 0.001 μl 1,8-cineole (approx. 3 x 10-8 M), the SCA of the bronchi remains virtually unchanged. Only a tendency to suppress the SCA in the effect with 0.005 μl 1,8-cineole (approx. 1.5 x 10-7 M) can be recorded (Figure. 4, bottom).
In Figure 4 (top) the inhibitory effects on the contractile activity of the bronchi are already clearly visible.
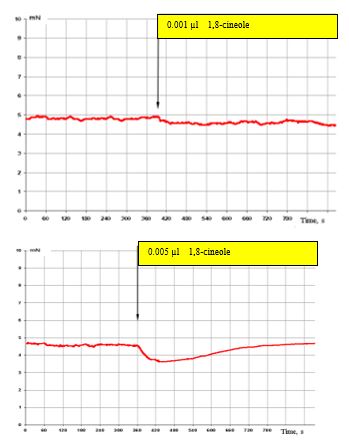
Figure 4: Effect of 0.001 μl 1,8-cineole (approx. 6 x 10-7 M/L) top, and 0.005 μl (bottom) on the contractile activity of smooth muscle fibre of human segmental bronchi.
In the case of a further increase in 1,8-cineole to 0.01 μl (3 x 10-7 M), a very pronounced suppression of the SCA of the bronchi is observed (Figure. 5, top), which however changes to a stimulating effect after 3-4 minutes. By contrast, in the case of the effect with 0.1 μl 1,8-cineole (approx. 3 x 10-5 M), only a pronounced stimulatory effect can be registered (Figure. 5, below). It must be taken into account that short-term effects with 1,8-cineole levels of 0.1 μl (3 x 10-5 M) lie below the necessary concentrations for a spasmolytic effect.
Furthermore, 0.01 μl 1,8-cineole (equivalent to a concentration of approx. 3 x 10–6 M) was in each case used to be able to explain the mentioned effects of 1,8-cineole on the contractile activity of the bronchi.
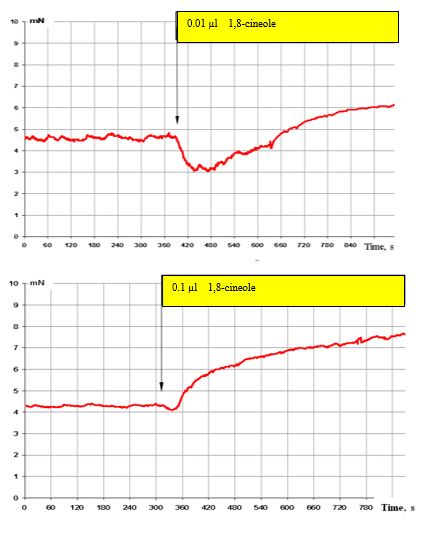
Figure 5: Effect of 0.01 μl (top) and 0.1 μl (bottom) on the contractile activity of smooth muscle fibre of human segmental bronchi.
Figure 6: (top) shows the effect of 0.01 μl 1,8-cineole on the SCA of bronchi and with prior administration of 5 x 10-6 M benextramine (α1 α2 adrenoreceptor blocker) (Figure. 6, bottom). Blocking of α1 and α2 adrenoreceptors completely cuts off the stimulating effect of 1,8-cineole on the SCA of the bronchi. Only the inhibitory components of these effects persist.
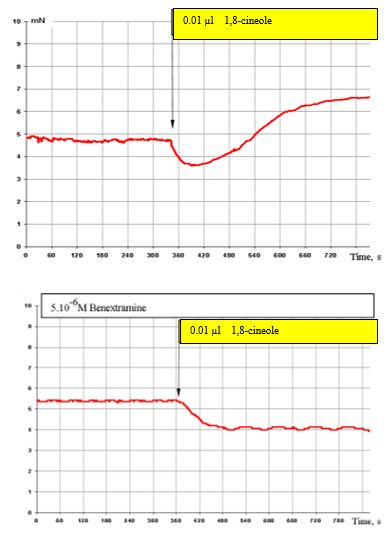
Figure 6: Effect of 0.01 μl 1,8-cineole on the contractile activity of smooth muscle fibre of human segmental bronchi under normal conditions (top) and with prior administration of 5 x 10-6 benextramine (bottom).
Figure 7: shows that the blocking of H2 histamine receptors completely cuts off the inhibitory components of the 1,8-cineole effect on the SCA of the bronchi. Only the stimulating effects of 1,8-cineole on the SCA of the bronchi remain.

Figure 7: Effect of 0.01 μl 1,8-cineole on the contractile activity of smooth muscle fibre of human segmental bronchi under normal conditions (top) and with prior administration of 5 x 10-6 M famotidine, a H2 histamine antagonist (bottom).
Figure 8: shows that the overall effect of 1,8-cineole with simultaneous blocking of α1, α2 adreno- and H2 histamine receptors is completely blocked.
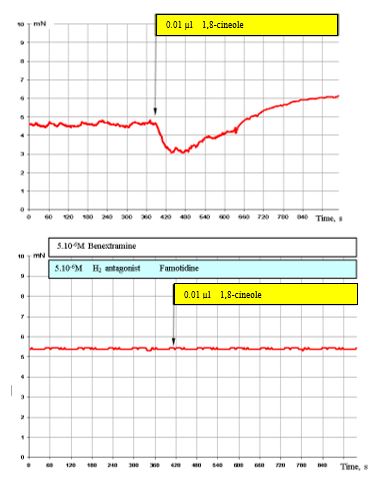
Figure 8: Effect of 0.01 μl 1,8-cineole on the contractile activity of smooth muscle fibre of human segmental bronchi (top) and with prior administration of 5 x 10-6 M benextramine and 5 x 10-6 M famotidine (bottom).
Figure 9: Shows the results of the effects of 10-6 MH1 histamine agonists on the SCA of the bronchi under normal conditions (top) and with prior administration of 0.005 μl 1,8-cineole (approx. 1.5 x 10-6 M). The stimulating effects of the H1 agonist (top) are clearly reduced (Figure. 9, bottom).
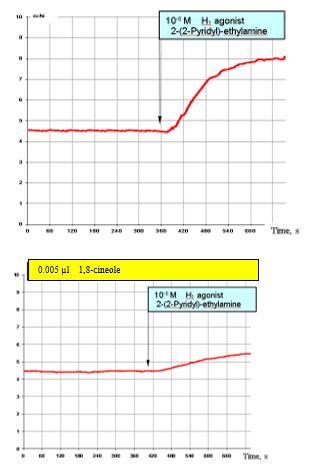
Figure 9: Effect of 10-6 M H1 agonist (2–(2 pyridil)-ethylamine) on the contractile activity of smooth muscle fibre of human segmental bronchi under normal conditions and with prior administration of 0.005 μl 1,8-cineole.
The effects of 10-5 M ACh under normal conditions (top) and with prior administration of 0.01 μl 1,8-cineole are presented in Figure 10. In the presence of 0.01 μl 1,8-cineole, the stimulating effects of ACh remain unchanged.
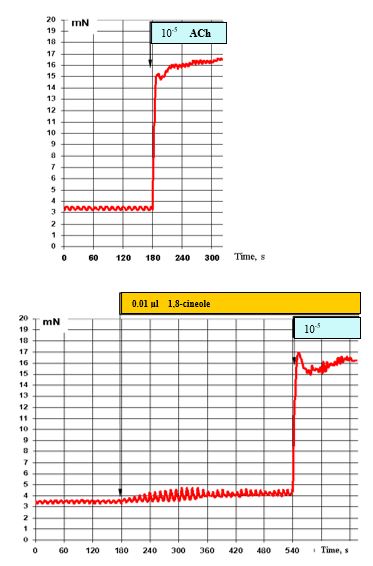
Figure 10: Effect of 10-5 M ACh on the contractile activity of smooth muscle fibre of human segmental bronchi (top) and with prior administration of 0.01 μl 1,8-cineole (bottom).
Figure 11: shows that the stimulating effects of ACh remain unchanged even after several effects of 0.01 μl 1,8-cineole.
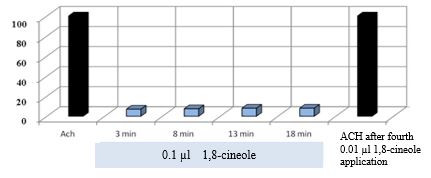
Figure 11: Effect of 10-5 M ACh on the contractile activity of smooth muscle fibre of human segmental bronchi at the start of the experiment and after 4x administration of 0.01 μl 1,8-cineole.
Discussion
1,8-cineole is a monoterpene known to be a component of various essential oils, e.g. of the genus Eucalyptus, Salvia, Rosmarinus, but is mainly isolated from Eucalyptus species which produce essential oil rich in cineole. This saturated terpene has a number of medicinally useful anti-inflammatory, anti-oxidative and antimicrobial effects, as recently presented in an overview by Juergens [6]. An experimental study provides useful data suggesting that 1,8-cineole is a specific COX-2 blocker [7]. 1,8-cineole is used as an active ingredient in medicinal products and can be inhaled, topically applied or be taken orally. After resorption of 1,8-cineole in the small intestine part of it is eliminated unchanged by exhalation [8]. In the intestinal as well as the bronchial tract, 1,8-cineole also comes into contact with smooth muscle fibre. Investigations of the effects of 1,8-cineole on SMF, whether receptor-specific or receptor-independent, are expected to contribute to understanding of the clinical efficacy of 1,8-cineole, e.g. in inflammatory bronchial diseases [9]. Our results show that the mechanisms of this inhibition are due to an effect specific to the human bronchial system. Biologically active substances can influence the contractile activity of smooth muscle in various ways. Firstly, the contractile activity of smooth muscle can be influenced by an effect on the receptors present on smooth muscle cells, e.g. through the effects of histamine, dopamine, acetylcholine, other mediators or with synthetic substances. For influences at the receptor level, the effects on the contractile activity of smooth muscle typically only occur after several seconds. The second notable aspect of these effects is that demonstrable changes in contractile activity are still observed at concentrations of 10-7 - 10-9 M. Very often, these are reversible.
Secondly, biologically active substances have effects on different enzyme systems (e.g. phosphodiesterases, adenylate cyclases, cyclooxygenases). For such substances, the effects typically occur only after several minutes and at concentrations above 10-6 M. Independent of other properties of a substance (spasmolytic effects etc.), these time and concentration intervals allow the effects of biologically active substances to be analysed at the receptor level. This in particular applies to substances exhibiting multiple effects on smooth muscle, such as, for example, some monoterpenes (1,8-cineole, thymol) with a very powerful spasmolytic effect. As a result, short-acting effects and effects at low concentrations, have allowed identification of previously unknown effects with 1,8-cineole and other compounds.
The stimulating effects of histamine continue to suppress the smooth muscle at 1,8-cineole concentrations of 3 x 10-6 M. This proves that the smooth muscle of the segmental bronchi possesses histamine H1 receptors.
1,8-cineole has an agonistic effect on histamine H2 receptors, causing very strong suppression of the contractile activity of smooth muscle. The smooth muscles of the bronchi also possess these receptors. It could be shown in the experiments that 1,8-cineole already exerts a powerful suppressive effect on the contractile activity of the bronchi at a concentration of 3 x 10-6 M.
It was also demonstrated that 1,8-cineole, at a concentration of 3 x 10-6 M, has agonistic effects on α1 und α2 adrenoreceptors [3]. Activation of α1 and α2 adrenoreceptors, which also occurs with other monoterpenes, e.g. with thymol, is observed at very high concentrations [10]. This is the reason for the existence of contradictory reports on the effect of 1,8-cineole on the bronchi. At a low doses, the stimulating effects of 1,8-cineole dominate through the activation of the α1 and α2 adrenoreceptors. Additional intensification of the bronchial spasmolytic properties results as a consequence of the mentioned effects. At somewhat higher concentrations, an equilibrium occurs between the stimulating and suppressive effects on the contractile activity of the bronchi. A pronounced spasmolytic effect on the bronchi is recorded at higher concentrations.
At sufficiently high concentrations of 1,8-cineole (approx. 10-5 M in vitro), contraction is blocked due to the stimulation of α1 and α2 adrenoreceptors by its spasmolytic effect, as there are insufficient free Ca2+ ions available. The spasmolytic effect of 1,8-cineole cannot influence the effects on the H1 and H2 histamine receptors and the blocking of cyclooxygenase. As a result, the desired 1,8-cineole effects are attained:
1. A specific blocker of H1 histamine receptors, with no effect on ACh receptors.
2. Inhibition of the contractile activity of human bronchial smooth muscle through activation of H2 histamine receptors.
These results are in line with former investigations indicating the spasmolytic effect of 1,8-cineole on SMF in our results [3]. A study by Wagner [11] showed, however, that terpenes, when administered to patients with bronchial asthma, resulted in paradoxical bronchoconstriction reactions. We believe that these contradictory reports are based on the special properties of terpenes: Monoterpenes are known to have both specific and non-specific effects on smooth muscle fibre, dependent on the dose. Some monoterpenes, such as 1,8-cineole and thymol [10], at concentrations of up to 5 x 10-7 M, lead to constricting effects on almost all smooth muscle fibre. These effects are due to the agonistic effects on α1 and α2 adrenoreceptors. However, they can only be observed up to an administered dose of a concentration of 5 x 10-7 M. Exceeding this amount, the pronounced spasmolytic effect of cineole appears, which no longer allows the registration of specific effects. Low concentrations of 1,8-cineole allow observations of bronchoconstriction. Here, we recorded the stimulating effects of 1,8-cineole on smooth muscle fibre (due to the excitation effects on H1 receptors). At higher concentrations very pronounced spasmolytic effects can be observed and, of course, inhibition of H1 receptor activity. The concentration-response curve of the effects of histamine indicates that the maximum excitation effects are achieved at a concentration of 5 x 10-6 M. The results of the interaction between 1,8-cineole and histamine indicate that 1,8-cineole is a reversible antagonist of histamine H1 receptors. Regarding the clinical relevance of the use of 1,8-cineole, the well-known spasmolytic effect of 1,8-cineole in therapeutic concentrations could be particularly valuable in inflammatory airway diseases with bronchoconstriction symptoms, which has already been confirmed in clinical trials with patients suffering from asthma or COPD [7].
Conclusions
The ultimate goal of this study was to address the bronchodilatory effect of this compound, using human airway smooth muscle in order to demonstrate a possible role for 1,8-cineole in airway diseases. Human airway smooth muscle is an appropriate substrate because the pharmacological properties of airway smooth muscle could differ in several aspects from those of gastric and other types of smooth muscle.
List of Abbreviations
Acetylcholine (ACh).
Krebs´ solution (KS)
Newtons (N)
Smooth muscle fibres (SMF)
Spontaneous contractile activity (SCA)
All experiments were approved by the Bulgarian Food Safety Agency (approval number: 21/19.03.2012) and the Ethics Committee of the Medical University of Plovdiv, Bulgaria (approval number: 2/13.03.2014).
Funding
Prof. J. Lukanov reported receiving financial support by Klosterfrau Company used for reagents and animals to perform the study. All other authors declare that they have no competing interests.
Acknowledgements
We thank the Klosterfrau Company for their financial support in making this study possible.
References
1. Jürgens UR, Vetter H. 1998. Terpene in der Asthmatherapie: Neue klinische und experimentelle Ergebnisse zur antiinflamatorischen und bronchodilatorischen Wirkungen von 1.8-Cineol. In: Loew D und N Rietbrock ( Hrsg.) Phytopharmaka IV Steinkopff Darmstadt. Ref.: https://bit.ly/2XwsIMe
2. Cabo J, Crespo ME, Jimenez J, et al. 1986. The spasmolytic activity of various aromatic plants from the province of Granada. The activity of the major components of their essential oils. Plantes Medicinales et Phythotherapie, 10: 213 -218.
3. Sagorchev P, Lukanov J, Beer A-M. 2015. Effect of 1.8-cineole (eucalyptole) on the spontaneous contractile activity of smooth muscles fibre. Journal of medicinal plants research. 9: 486-493. Ref.: https://bit.ly/343ccWy
4. Beer AM, Sagorchev P, Uzunova J, et al. 2017. Effects of 1.8-cineole (eucalyptol) on the Activity of Histamine H1 Receptors. SF J Plant Physiol. 1:1. Ref.: https://bit.ly/2reiWCk
5. Golenhofen K, Bülbring E, Shuba MF. 1976. Spontaneous Activity and functional classification of mammalian smooth muscle. In: eds. Physiology of smooth muscle. New York: Raven Press. 91-97.
6. Juergens UR. 2014. Anti-inflammatory Properties of the Monoterpene 1.8-cineole: Current Evidence for Co-medication in Inflammatory Airway Diseases. Drug Res 64:1-9. Ref.: https://bit.ly/2KI5Zrr
7. Beer AM, Zagorchev P, Filipova DM, et al. 2017. Effects of 1.8-Cineole on the Activity of Cyclooxygenase and Cyclooxygenase 1 and Cyclooxygenase 2 Isoforms. Nat Prod Chem Res. 5: 253. Ref.: https://bit.ly/2XwublI
8. Beauchamp J, Kirsch F, Buettner A. 2010. Real-time breath gas analysis for pharmacokinetics: monitoring exhaled breath by on-line proton-transfer-reaction mass spectrometry after ingestion of eucalyptol-containing capsules. J. Breath Res. 4: 026006 (12pp). Ref.: https://bit.ly/332iPY1
9. Wittmann M, Petro W, Kaspar P, et al. 1998. Therapy with expectorants: a double-blind randomised study comparing ambroxol and cineole (in German). Atemw-Lungenkrkh. 24: 67-74.
10. Beer AM, Lukanov J, Zagortchev P. 2007. Wirkung von Thymol auf die kontraktile Aktivität der glatten Muskulatur. Phytomedicine. 14: 65-69. Ref.: https://bit.ly/35wc6r1
11. Wagner H. 1985. Pharmazeutische Biologie 2: Terpene und Inhaltsstoffe, 3. Aufl., Gustav Fischer, Stuttgart. New York. 62-63.




















