Indexing & Abstracting
Full Text
Review ArticleDOI Number : 10.36811/ojpsr.2022.110016Article Views : 0Article Downloads : 3
Study of the relationship between the mortality rate among patients with covid-19 and diabetes in al-bieda city
Eman GA Allafi1*, Afya M Jadallah1, Azza M Mohammad1, Aziza M Abdallah1 and Fayrouz A Khaled2
1Biomedical Science Department, Faculty of pharmacy, Omar Al-Mokhtar University, Libya
2Department of Chemistry, Faculty of Science, Omar Al-Mokhtar University, El-Beida, Libya
*Corresponding Author: Eman GA Allafi, Biomedical Science Department, Faculty of pharmacy, Omar Al-Mokhtar University, Libya; Email: fayalzobair@yahoo.com
Article Information
Aritcle Type: Review Article
Citation: Eman GA Allafi, Afya M Jadallah, Azza M Mohammad, et al. 2022. Study of the relationship between the mortality rate among patients with covid-19 and diabetes in al-bieda city. Open J Pharm Sci Res. 4: 01-05.
Copyright: This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Copyright © 2022; Eman GA Allafi
Publication history:
Received date: 07 September, 2022Accepted date: 21 September, 2022
Published date: 23 September, 2022
Abstract
Background and Aim: We have conducted a study on COVID-19 patients with diabetes who developed diabetes after infection with a virus and compared between diabetic and non-diabetic cases in terms of the effect of a virus on them and the mortality rate among them.
Methods: A cross-sectional study of COVID-19 patients in which 180 male and female cases, aged between 36 and 96 years, were reported. The collected data were analyzed and the outcome results were displayed in tables and charts. The diabetic patients, as noticed from charts and the calculation that were done by the research team, were more susceptible to die than non-diabetic patients of the near age.
Keywords: COVID 19; Diabetes; Libya; Patients
Introduction
There's a bidirectional relationship between Covid-19 and diabetes. On the one hand, diabetes is related with an expanded hazard of extreme Covid-19. On the other hand, newonset diabetes and extreme metabolic complications of preexisting diabetes, counting diabetic ketoacidosis and hyperosmolarity for which outstandingly tall measurements of affront are justified, have been watched in patients with Covid-19 [1-3]. These appearances of diabetes posture challenges in clinical administration and recommend a complex pathophysiology of Covid-19–related diabetes. Serious intense respiratory disorder coronavirus 2 (SARS-CoV-2), the infection that causes Covid-19, ties to angiotensinconverting chemical 2 (ACE2) receptors, which are communicated in key metabolic organs and tissues, counting pancreatic beta cells, fat tissue, the little digestive tract, and the kidneys [4]. In this way, it is conceivable that SARSCoV-2 may cause pleiotropic modifications of glucose digestion system that might complicate the pathophysiology of preexisting diabetes or lead to modern components of malady. These are moreover a few points of reference for a viral cause of ketosis-prone diabetes, counting other coronaviruses that tie to ACE2 receptors [5]. More noteworthy rates of fasting glycemia and acute-onset diabetes have been detailed among patients with SARS coronavirus-1 pneumonia than among those with non-SARS Pneumonia [5]. RT-PCR For intense respiratory illnesses and utilized for patients with COVID19 [6]. Beat oximetry Oxygen level drops for COVID-19 patients without respiratory side effects [7]. ABG Arrange in patients with serious ailment as demonstrated to distinguish hypercarbia or acidosis. Prescribed in patients with respiratory trouble and cyanosis who have moo oxygen immersion (SpO? <90%). The number of CD4+ and CD8+ T cells diminished in those enduring from incessant diseases [8]. Thyroid work test for constant illness Moo levels in a few patients of triiodothyronine (T3) and typical or moo thyroid-stimulating hormone (TSH) [9]. Which we focused on in our research to find out the extent of its impact on patients with COVID-19 and its levels, its relationship to mortality Test for chronic diseases Fasting hyperglycemia independently has a poor prognosis and is associated with increased mortality, whether or not the patient has diabetes [10,11]. Hyperglycemia, the core feature of diabetes, is associated with inflammation and weakened immunity against infections, and was recognised as a significant risk factor for severe Covid-19 early in the pandemic. Serum C- reactive protein was elevated in severe cases [12,13]. Procedure for patients suspected of having pneumonia. Some studies said that chest infection correct for 80.6% patients with COVID-19, although 28% were diagnosed as not infected, but they were infected with COVID-19 [14]. Chest CT is sensitive and moderately specific for the diagnosis of COVID?19. Pooled results found that chest CT correctly diagnosed COVID?19 in 87.9% of people who had the disease. However, it incorrectly identified COVID?19 in 20% of people who did not have the disease [14]. Lung ultrasound is used as a diagnostic tool in some centers as an alternative to chest x-ray and chest CT [15] . D-dimer COVID-19, the pandemic disease caused by infection with the novel virus, SARS-CoV-2 (severe acute respiratory syndrome coronavirus-2) can now be added to the already extensive list of conditions that may be associated with elevated D-dimer.
Material & Methods
A cross-sectional study for the years 2021- 2022, 180 male and female cases were studied in Mansoura Hospital specialized in chest diseases, which is located in Jabal Al-Akhdar in Libya, located 20 km north-east of Al-Bayda city and north of the city of Shahat. The data were obtained from the hospital archive. All the tests, including PCR and chest X-rays, were carried out in the local laboratories.
|
Table 1: The cases understudy grouped in intervals. |
|||
|
Age intervals |
Patients |
Diabetic |
Non- diabetic |
|
30-40 |
6 |
2 |
4 |
|
41-50 |
25 |
8 |
17 |
|
51-60 |
28 |
19 |
9 |
|
61-70 |
42 |
25 |
17 |
|
71-80 |
53 |
25 |
28 |
|
81-90 |
17 |
8 |
9 |
|
91-105 |
7 |
2 |
5 |
|
D: diabetic, ND: nondiabetic |
|||
Result and Discussion
A cross-sectional study of COVID-19 patients in which 180 male and female cases, aged between 36 and 96 years, were reported. The collected data were analyzed and the outcome results were displayed in tables and charts. The diabetic patients, as noticed from charts and the calculation that were done by the research team, were more susceptible to die than non-diabetic patients of the near age. From the previous table, 50% of the cases are diabetics. The data analysis, displayed in the figures I and II, shows that the severity and the mortality rate in the non-diabetic covid-19 patient increases with age. On the other hand, corona patient with chronic diseases, especially diabetes, the mortality percentage highly affected with general health status of the patients. The exhausted body and weak immunity system in patients of chronic diseases share the responsibility with the invading viruses of the escalating mortality rate.
Intravenous fluids, World Health Organization guidelines recommend that patients with COVID-19 in respiratory failure should be treated cautiously with intravenous fluids, especially in settings with limited availability of mechanical ventilation Hydroxychloroquine (HCQ) Chloroquine, a widely used antimalarial has been reported as a potential broadspectrum antiviral drug [16,17] . Chloroquine blocks viral infections by increasing endosomal pH which then interferes with virus/cell fusion. This drug also interferes with the glycosylation of cellular receptors for SARS-CoV and hence decreases virus-cell binding [18]. Zinc, as an important micronutrient, plays a key role in macronutrient metabolism as well as appetite control [19] . In addition, zinc is involved in synthesis, storage, release, and action of insulin [20,21] and its deficiency is associated with insulin resistance, impaired glucose tolerance and obesity [22,23].

Figure 1: Percentage of dead non diabetic.
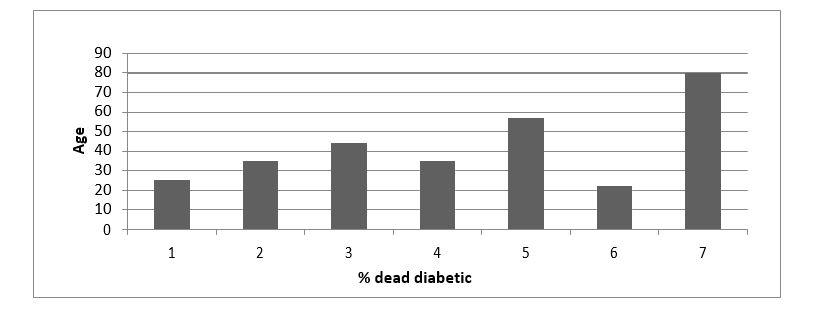
Figure 2: Percentage of dead diabetic.
Conclusion
Corona patient with chronic diseases, especially diabetes, the mortality percentage highly affected with general health status of the patients. The exhausted body and weak immunity system in patients of chronic diseases share the responsibility with the invading viruses of the escalating mortality rate.
References
1. Cristelo C, Azevedo C, Marques JM, et al. 2020. SARS-CoV-2 and diabetes: New challenges for the disease. Diabetes Research and Clinical Practice. 164: 108228. Ref.: https://pubmed.ncbi.nlm.nih.gov/32446801/ DOI: https://doi.org/10.1016/j.diabres.2020.108 228
2. Conte G, Cei M, Evangelista I, et al. 2021. The meaning of D-dimer value in COVID19. Clinical and Applied Thrombosis/Hemostasis. 27: 10760296211017668. Ref.: https://pubmed.ncbi.nlm.nih.gov/34011194/ DOI: https://doi.org/10.1177/10760296211017668
3. Berger JS, Kunichoff D, Adhikari S, et al. 2020. Prevalence and outcomes of D-dimer elevation in hospitalized patients with COVID-19. Arteriosclerosis, thrombosis, and vascular biology. 40: 2539-2547. Ref.: https://pubmed.ncbi.nlm.nih.gov/32840379/ DOI: https://doi.org/10.1161/atvbaha.120.314872
4. Mantovani A, Byrne CD, Zheng MH, et al. 2020. Diabetes as a risk factor for greater COVID-19 severity and in-hospital death: a meta-analysis of observational studies. Nutrition, Metabolism and Cardiovascular Diseases. 30: 1236-1248. Ref.: https://pubmed.ncbi.nlm.nih.gov/32571616/ DOI: https://doi.org/10.1016/j.numecd.2020.05. 014
5. Zeyaullah M, AlShahrani AM, Muzammil K, et al. 2021. COVID-19 and SARS-CoV2 variants: current challenges and health concern. Frontiers in Genetics. 12: 693916. Ref.: https://pubmed.ncbi.nlm.nih.gov/34211506/ DOI: https://doi.org/10.3389/fgene.2021.693916
6. World Health Organization. 2021. Antigendetection in the diagnosis of SARS-CoV-2 infection: interim guidance.
7. Xie J, Tong Z, Guan X, et al. 2020. Critical care crisis and some recommendations during the COVID-19 epidemic in China. Intensive care medicine. 46: 837-840. Ref.: https://pubmed.ncbi.nlm.nih.gov/32123994/ DOI: https://doi.org/10.1007/s00134-020- 05979-7
8. Huang W, Berube J, McNamara M, et al. 2020. Lymphocyte subset counts in COVID?19 patients: a meta?analysis. Cytometry. 97: 772-776. Ref.: https://pubmed.ncbi.nlm.nih.gov/32542842/ DOI: https://doi.org/10.1002/cyto.a.24172
9. Malik J, Zaidi SMJ, Waqar AU, et al. 2021. Association of hypothyroidism with acute COVID-19: A systematic review. Expert Review of Endocrinology & Metabolism. 16: 251-257. Ref.: https://pubmed.ncbi.nlm.nih.gov/34424110/ DOI: https://doi.org/10.1080/17446651.2021.19 68830
10. Lazarus G, Audrey J, Wangsaputra VK, et al. 2021. High admission blood glucose independently predicts poor prognosis in COVID-19 patients: a systematic review and dose-response meta-analysis. Diabetes research and clinical practice. 171: 108561. Ref.: https://pubmed.ncbi.nlm.nih.gov/33310127/ DOI: https://doi.org/10.1016/j.diabres.2020.108 561
11. Handayani DR, Juliastuti H, Nawangsih EN, et al. 2021. Prognostic value of fasting hyperglycemia in patients with COVID19–Diagnostic test accuracy meta-analysis. Obesity medicine. 23: 100333. Ref.: https://pubmed.ncbi.nlm.nih.gov/33842733/ DOI: https://doi.org/10.1016/j.obmed.2021.100333
12. Danwang C, Endomba FT, Nkeck JR, et al. 2020. A meta-analysis of potential biomarkers associated with severity of coronavirus disease 2019 (COVID-19). Biomarker research. 8: 1-13. Ref.: https://pubmed.ncbi.nlm.nih.gov/32879731/ DOI: https://doi.org/10.1186/s40364-020- 00217-0
13. Yitbarek GY, Walle Ayehu G, Asnakew S, et al. 2021. The role of C-reactive protein in predicting the severity of COVID-19 disease: A systematic review. SAGE Open Medicine. 9: 20503121211050755. Ref.: https://pubmed.ncbi.nlm.nih.gov/34659766/ DOI: https://doi.org/10.1177/20503121211050755
14. Islam N, Ebrahimzadeh S, Salameh JP, et al. 2021. Thoracic imaging tests for the diagnosis of COVID?19. Cochrane Database of Systematic Reviews. 3. Ref.: https://pubmed.ncbi.nlm.nih.gov/3372444 3/ DOI: https://doi.org/10.1002/14651858.cd01363 9.pub4
15. World Health Organization. 2020. Use of chest imaging in COVID-19: a rapid advice guide. World Health Organization.
16. Savarino A, Di Trani L, Donatelli I, et al. 2006. New insights into the antiviral effects of chloroquine. The Lancet infectious diseases. 6: 67-69. Ref.: https://pubmed.ncbi.nlm.nih.gov/16439323/ DOI: https://doi.org/10.1016/s1473- 3099(06)70361-9
17. Yan Y, Zou Z, Sun Y, et al. 2013. Antimalaria drug chloroquine is highly effective in treating avian influenza A H5N1 virus infection in an animal model. Cell research. 23: 300-302. Ref.: https://pubmed.ncbi.nlm.nih.gov/23208422/ DOI: https://doi.org/10.1038/cr.2012.165
18. Vincent MJ, Bergeron E, Benjannet S, et al. 2005. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virology. 2: 1-10. Ref.: https://pubmed.ncbi.nlm.nih.gov/16115318/ DOI: https://doi.org/10.1186/1743-422x-2- 69
19. Song Y, Wang J, Li XK, et al. 2005. Zinc and the diabetic heart. Biometals. 18: 325- 332. Ref.: https://pubmed.ncbi.nlm.nih.gov/16158224/ DOI: https://doi.org/10.1007/s10534-005- 3689-7
20. Simon SF, Taylor CG. 2001. Dietary zinc supplementation attenuates hyperglycemia in db/db mice. Experimental Biology and Medicine. 226: 43-51. Ref.: https://pubmed.ncbi.nlm.nih.gov/11368237/ DOI: https://doi.org/10.1177/153537020122600 107
21. Arthur BC, Chausmer S. 1998. Zinc, insulin and diabetes. J Am Coll Nutri. 17: 109-115. Ref.: https://pubmed.ncbi.nlm.nih.gov/9550453/ DOI: https://doi.org/10.1080/07315724.1998.10 718735
22. Tallman DL, Taylor CG. 2003. Effects of dietary fat and zinc on adiposity, serum leptin and adipose fatty acid composition in C57BL/6J mice. The Journal of nutritional biochemistry. 14: 17-23. Ref.: https://pubmed.ncbi.nlm.nih.gov/1255947 3/ DOI: https://doi.org/10.1016/s0955- 2863(02)00228-0
23. Marreiro DDN, Fisberg M, Cozzolino SMF. 2002. Zinc nutritional status in obese children and adolescents. Biological trace element research. 86: 107-122. Ref.: https://pubmed.ncbi.nlm.nih.gov/12008974/ DOI: https://doi.org/10.1385/bter:86:2:107




















