Indexing & Abstracting
Full Text
Review ArticleDOI Number : 10.36811/ojfnr.2019.110001Article Views : 2883Article Downloads : 38
Shedding new light on cancer management - a renewed focus on cancer cachexia
Ling Hui Claytor
Asia Pacific Medical Director, Abbott Nutrition, Singapore
*Corresponding author: Dr. Ling Hui Claytor, Asia Pacific Medical Director, Abbott Nutrition, 3 Fraser Street, Duo Tower, Singapore, Email: huilinghuiling@yahoo.com
Article Information
Aritcle Type: Review Article
Citation: Ling Hui Claytor. 2019. Shedding new light on cancer management - a renewed focus on cancer cachexia. Open J Food Nutri Res. 1: 01-07.
Copyright: This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Copyright © 2019; Ling Hui Claytor
Publication history:
Received date: 15 February, 2019Accepted date: 25 February, 2019
Published date: 27 February, 2019
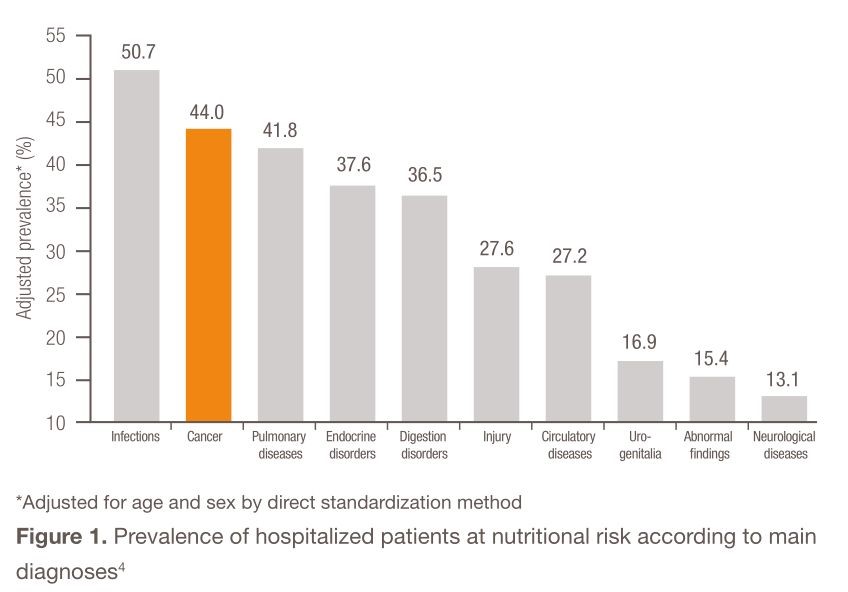
Malnutrition in cancer patients has been well documented and studied for the past 40 years [1]. In the landmark study by Dewys and colleagues involving over 3000 patients enrolled in Eastern Cooperative Oncology Group (ECOG) chemotherapy trials, moderate-to-severe weight loss was reported in 30–70% of cancer patients, with the greatest incidence found in those with solid tumors along the upper gastrointestinal tract and in the lungs [2]. Decades later, in a 2009 study by Bozzetti et al. analyzing the nutritional status of 1000 outpatients, 40% of cancer patients were observed to have lost over 10% of their body weight [3]. Cancer patients were one of the most nutritionally at-risk populations compared with other hospitalized patients [4] (Figure 1).
The wasting syndrome commonly observed in cancer patients is defined as cancer cachexia, a multifactorial condition characterized by continuous loss of skeletal muscle mass, with or without loss of adipose tissue. It is driven largely by reduced food intake and altered metabolism, but cannot be completely treated with conventional nutritional support [5]. Numerous studies have shown that cancer patients who experience weight loss or cachexia have significantly poorer survival outcomes, functional status, and quality of life, while having poor response to chemotherapy and a higher risk for chemotherapy toxicity [1,6]. Between 4% and 23% of patients with terminal cancer will eventually die as a result of cachexia [6]. Despite the considerable prevalence and severe consequences, weight loss in cancer patients is seldom recognized, systematically assessed, or actively managed [7,8]. Furthermore, the majority of cancer patients appear physically to be of normal weight, and fewer than 7% have obvious malnutrition (body mass index <18.5 kg/m2) [1].
Early nutritional intervention matters
Patients who are referred to specialized units for nutritional support typically present with advanced cachexia, which is usually resistant to any intervention. This underlines the importance of identifying and assessing nutritionally at-risk individuals for early multimodal interventions before cachexia becomes refractory and difficult to reverse [5,6].
Nutrition support therapy should thus be offered to patients who are malnourished, at nutritional risk, or likely to develop anorexia or gastrointestinal defects due to treatment side effects [6,9]. These patients may be identified using nutritional screening tools, including the Patient Generated Subjective Global Assessment (PG-SGA) questionnaire, the Nutrition Risk Index (NRI), the Malnutrition Universal Screening Tool (MUST), and the Nutritional Risk Screening 2002 (NRS-2002) system [6,9]. The first step of nutrition therapy should be counseling by a trained dietitian to encourage consumption of foods and fluids rich in energy and protein [9]. Oral nutritional supplement is recommended when nutritional goals are not met on an enriched diet.9 Supplementation with omega-3 fatty acids such as eicosapentaenoic acid may help stabilize weight in cancer patients on oral diets experiencing continuous unintentional weight loss [10]. Regular physical activity, under appropriate supervision or through exercise programs, should be encouraged to preserve muscle mass and support physical performance [9]. This article aims to explain the role of nutrition therapy in the prevention of cachexia and management of malnutrition in cancer patients.
Management of cancer cachexia: Updates in nutrition therapy
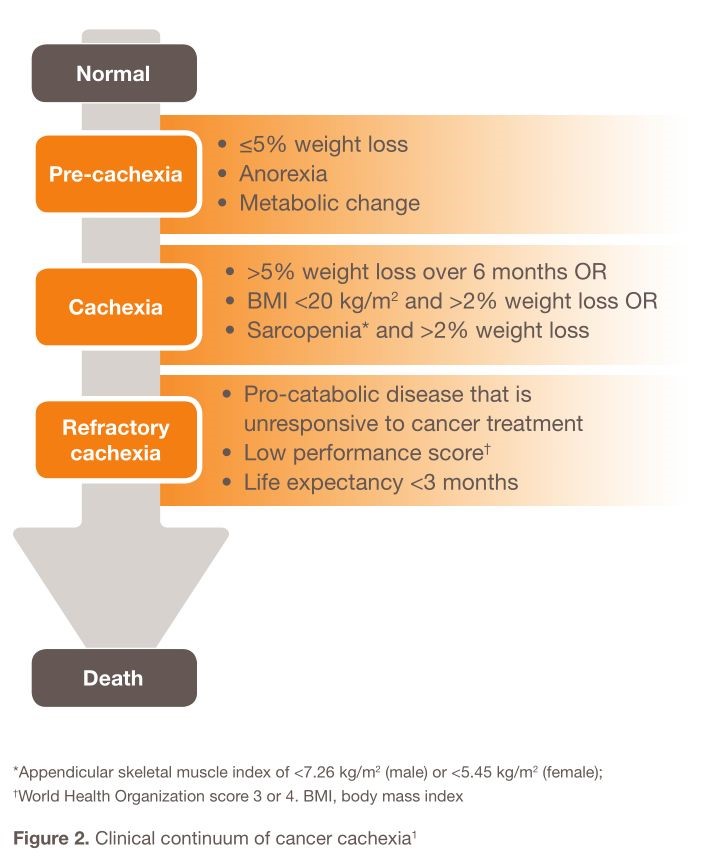
Cancer cachexia is defined as a “multifactorial syndrome characterized by an ongoing loss of skeletal muscle mass (with or without loss of fat mass) that cannot be fully reversed by conventional nutritional support, leading to progressive functional impairment [11]”. Cancer cachexia is a continuous clinical spectrum that can be divided into three stages: pre-cachexia, cachexia, and refractory cachexia (Figure 2). The risk for progression is dependent on factors such as cancer type and stage, presence of systemic inflammation, level of food intake, and response to cancer treatment [11]. Cancer cachexia is the main reason that patients with cancers cannot tolerate the treatment cycles rather than pain itself.
Updated ESPEN guidelines on cancer nutrition
Issued in 2016, the European Society for Clinical Nutrition and Metabolism (ESPEN) guidelines on nutrition in cancer patients was developed with the aim of translating current best evidence and expert opinion into clinical recommendations for multidisciplinary teams in order to improve early detection and management of malnutrition in cancer patients [12]. From the time of cancer diagnosis, it is recommended that patients should be screened and assessed regularly for the risk for or presence of malnutrition. Adequate nutritional intake should be guided by a nutritional regimen in accordance with recommended energy and substrate requirements [12]. In order to meet these recommended requirements, the ESPEN guidelines advocate a stepwise approach when implementing nutrition therapy, from counseling to parenteral nutrition (Table 1) [12].
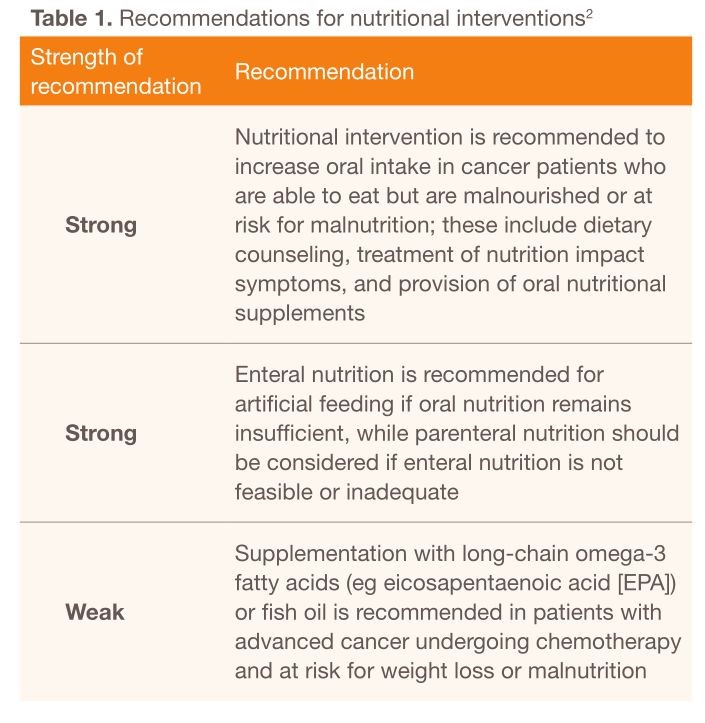
Notably, long-chain omega-3 fatty acids such as EPA (1–2 g/day) or fish oil (4–6 g/day) have been recommended for patients with advanced cancer to improve appetite, food intake, body weight, and lean body mass [12]. Omega-3 fatty acid supplementation has also been recommended for weight-losing cancer patients on oral diets in the 2009 American Society for Parenteral and Enteral Nutrition (ASPEN) guidelines [13]. Decisions on nutritional interventions should balance the expected benefits with the associated risks, burdens, and costs. Furthermore, nutritional therapy should be accompanied by resistance and aerobic exercise training to support muscle mass, physical performance, and metabolic pattern [12].
Multimodal management of cancer: Implementing parallel nutrition pathways
Nutritional deterioration has traditionally been considered an inextricable consequence of cancer. Even though numerous studies have established that weight loss is an independent prognostic factor of reduced survival, there remains limited awareness among oncologists on the importance of supporting patients’ nutritional status alongside treating the cancer itself [14].
As such, Muscaritoli et al. proposed a nutritional and metabolic approach in parallel with the oncology treatment pathway in the management of cancer patients. The ‘parallel pathway’ aims to provide early recognition, prevention, and treatment of cancer related malnutrition before the onset of cachexia [15]. The ‘parallel pathway’ approach involves multidisciplinary teams comprising physicians, dietitians, nurses, and psychologists. Within 4 weeks of cancer diagnosis, patients should undergo nutritional screening and assessment. A nutritional plan should then be drawn up, tailored to the patient’s needs and treatment plan proposed by the oncologist. The implementation of first-level nutritional intervention involving individualized oral diet and/or supplements should coincide with firstline cancer therapy. Follow-ups and reevaluations would guide subsequent nutritional strategies as patients undergo further cancer treatment [15].
The need to normalize metabolic changes
Randomized controlled trials have demonstrated that conventional nutritional support cannot completely treat cachexia [16]. Loss of weight and muscle is not solely due to insufficient food intake, but also caused by the activation of catabolic pathways mediated by pro-inflammatory cytokines, cortisol, and proteolysis-inducing factors [16,17]. Accumulating evidence has suggested that systemic inflammation related to cancer is a pivotal mediator for the progression of cancer cachexia and several pro- inflammatory cytokines released by both tumors and the host’s immune system including interleukins (1L) 1,2, and 6, interferon γ and TNF -α have been implicated in the pathogensis of cachexia in cancers.18 These factors depress appetite and impair the body’s metabolism of dietary fat, protein, and carbohydrate. Such changes actually begin before weight loss is evident and can worsen as cancer progresses.Nutritional interventions should thus incorporate antiinflammatory nutritional agents such as omega- 3 fatty acids (eg eicosapentaenoic acid [EPA]) or fish oil as a potential means of normalizing metabolic derangements that prevent weight gain in cancer patients [19,20]. Eicosapentaenoic acid (EPA) has been shown to help decrease the harmful metabolic changes induced by tumor-related factors. Calorically dense with a high amount of protein, oral nutritional supplement helps build lean body mass in weight-losing people with solid tumors. EPA, together with protein- and energy-rich ingredients helps counter the physiological abnormalities that underlie weight loss due to cancer cachexia.
Eicosapentaenoic acid in oral nutritional supplement
In a double-blind, randomized, placebo-controlled trial, 40 patients with stage III non-small-cell lung cancer (NSCLC) received either oral nutritional supplement (ONS) containing omega-3 fatty acids (2.0 g EPA + 0.9 g/day docosahexaenoic acid [DHA]) or an isocaloric control ONS [21]. Patients receiving ONS containing omega-3 fatty acids had better body-weight maintenance than patients on control supplement, with regression coefficients (β) of 1.1 kg (P=0.07), 1.3 kg (P=0.02), and 1.7 kg (P=0.04) after weeks 1, 2, and 4, respectively. Although fat-free mass in both groups declined, patients in the omega-3 fatty acids intervention group reported less loss than the control group at weeks 3 and 5 (β=1.5 kg, P=0.05; β=1.9 kg, P=0.02, respectively) [21].
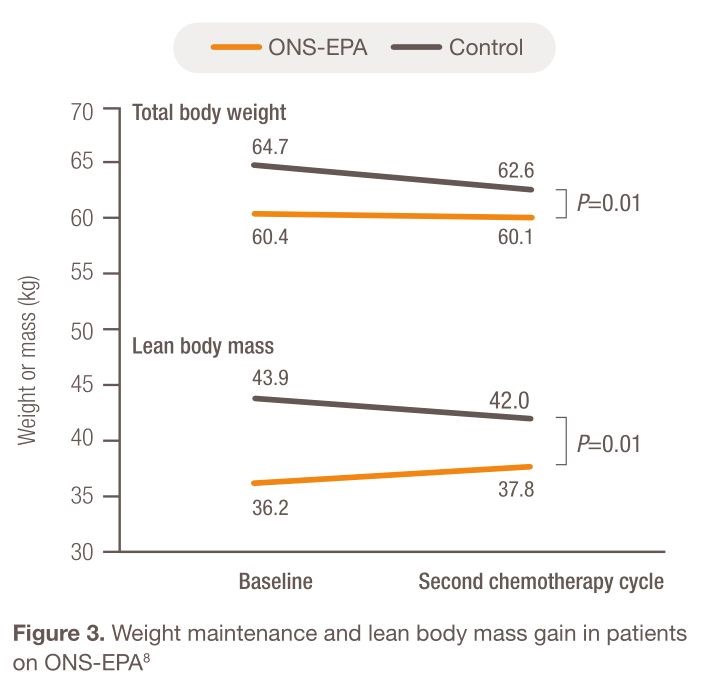
The effect of EPA was further studied in 92 patients with stage IIIb and IV NSCLC who were randomized to receive either standardized diet plus ONS-containing EPA (ONS-EPA) or isocaloric diet only.8 Patients receiving ONS-EPA maintained weight and gained 1.6±5 kg of lean body mass after two cycles of chemotherapy, while patients on the control diet sustained weight loss, losing 2.0±6 kg of lean body mass (Figure 3). Compared with baseline, there were also significant improvements in global, functional, and symptom scales of health-related quality of life in the ONS-EPA group as measured by global health status (P=0.02), fatigue (P=0.05), and loss of appetite (P=0.05). On the other hand, patients in the control group experienced significantly increased nausea and vomiting (P=0.02) and neuropathy (P=0.004). Diarrhea frequency was not significantly increased with the administration of ONS-EPA (P=0.19) [22].
Eicosapentaenoic acid in peri-operative enteral nutrition
The beneficial effects of EPA as part of peri-operative enteral nutrition were also observed in patients undergoing esophagectomy in a prospective, double-blind, randomized controlled trial. Fifty-three patients were administered either EPA-enriched enteral nutrition (2.2 g EPA/day) or isocaloric control formula without EPA [23]. EPA patients maintained lean body mass across all body segments post-operatively (P=0.8), while patients on the control formula lost 1.9±3.7 kg of fat-free mass (P=0.03, 95% CI 0.17, 3.6). Furthermore, stress response for tumor necrosis factor, interleukin 10, and interleukin 8 were significantly attenuated in the EPA arm (P<0.05) compared with the standard nutrition arm [23]. As inadequate nutritional intake and systemic inflammation syndrome are both commonly observed in cancer patients with cachexia, nutrition therapy should not only meet energy and protein requirements, but anti-inflammatory agents such as EPA should also be incorporated, along with exercise training [24]. In conclusion, nutrition is the most essential component of cancer management. If patients are unable to obtain adequate nutrition, the efficacy of any cancer treatment will be impaired. Both patients and health care professionals should therefore be educated to recognize the importance of nutrition and its effects on the efficacy of cancer treatment.
References
- Ryan AM, Derek G. Power, Louise Daly et al. 2016. Cancer-associated malnutrition, cachexia and sarcopenia: the skeleton in the hospital closet 40 years later. Proc Nutr Soc. 75: 199-211. [Ref.]
- Dewys WD, Begg C, Lavin PT, et al.1980. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am J Med. 69: 491-497. [Ref.]
- Bozzetti F, SCRINIO Working Group. 2009. Support Care Cancer. 17: 279-284. [Ref.]
- Tangvik RJ, Tell GS, Guttormsen AB et al. Nutritional risk profile in a university hospital population. Clin Nutr. 34 :705-711. [Ref.]
- Fearon K, Strasser F, Anker SD, et al. 2011. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 12: 489-495. [Ref.]
- Bozzetti F. 2013. Nutritional support of the oncology patient. Crit Rev Oncol Hematol. 87: 172-200. [Ref.]
- Churm D, Andrew IM, Holden K et al. A questionnaire study of the approach to the anorexia-cachexia syndrome in patients with cancer by staff in a district general hospital.Support Care Cancer. 17: 503-507. [Ref.]
- Spiro A, Baldwin C, Patterson A, et al. 2006. The views and practice of oncologists towards nutritional support in patients receiving chemotherapy. Br J Cancer. 95: 431-434. [Ref.]
- Arends J et al. Clin Nutr 2016: 1-38. [Ref.]
- August DA, Huhmann MB. 2009. A.S.P.E.N. clinical guidelines: nutrition support therapy during adult anticancer treatment and in hematopoietic cell transplantation. JPEN J Parenter Enteral Nutr. 33: 472-500. [Ref.]
- Fearon K, Strasser F, Anker SD, et al. 2011. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol.12: 489-495. [Ref.]
- Arends J et al. Clin Nutr 2016: 1-38.[Ref.]
- August DA, Huhmann MB. 2009. A.S.P.E.N. clinical guidelines: nutrition support therapy during adult anticancer treatment and in hematopoietic cell transplantation. JPEN J Parenter Enteral Nutr. 33: 472-500. [Ref.]
- Bozzetti F. 2013. Nutritional support of the oncology patient. Crit Rev Oncol Hematol. 87: 172-200. [Ref.]
- Muscaritoli M, Molfino A, Gioia G, et al. 2011. The "parallel pathway": a novel nutritional and metabolic approach to cancer patients. Intern Emerg Med. 6: 105-112. [Ref.]
- Ryan AM, Power DG2, Daly L, et al. 2016. Cancer-associated malnutrition, cachexia and sarcopenia: the skeleton in the hospital closet 40 years later. Proc Nutr Soc. 75: 199-211. [Ref.]
- Tisdale MJ. 2002. Cachexia in cancer patients. Nat Rev Cancer. 2: 862-871. [Ref.]
- Shirai Y et al. Nature Science Report 2017; 7: 4826-4835.[Ref.]
- Barber MD, Fearon KC, Tisdale MJ, et al. 2001. Effect of a fish oil-enriched nutritional supplement on metabolic mediators in patients with pancreatic cancer cachexia. Nutr Cancer. 40: 118-124. [Ref.]
- Barber MD, McMillan DC, Preston T, et al. 2000. Metabolic response to feeding in weight-losing pancreatic cancer patients and its modulation by a fish-oil-enriched nutritional supplement. Clin Sci (Lond). 98: 389-399. [Ref.]
- van der Meij BS, Langius JA, Smit EF, et al. 2010. Oral nutritional supplements containing (n-3) polyunsaturated fatty acids affect the nutritional status of patients with stage III non-small cell lung cancer during multimodality treatment. J Nutr. 140: 1774-1780. [Ref.]
- Sánchez-Lara K, Turcott JG1, Juárez-Hernández E, et al. 2014. Effects of an oral nutritional supplement containing eicosapentaenoic acid on nutritional and clinical outcomes in patients with advanced non-small cell lung cancer: randomised trial.Clin Nutr. 33: 1017-1023. [Ref.]
- Ryan AM, Reynolds, John V. MD, et al. 2009. Enteral nutrition enriched with eicosapentaenoic acid (EPA) preserves lean body mass following esophageal cancer surgery: results of a double-blinded randomized controlled trial. Ann Surg. 249: 355-363. [Ref.]
- Molfino A, Formiconi A, Rossi Fanelli F, et al. 2014. Cancer cachexia: towards integrated therapeutic interventions.Expert Opin Biol Ther. 14: 1379-1381. [Ref.]




















