Indexing & Abstracting
Full Text
Research ArticleDOI Number : 10.36811/ojfnr.2019.110003Article Views : 334Article Downloads : 318
Microencapsulation of Betalain from Philippine Beta vulgaris as stable colorant powder
Rosalinda C. Torres*, Rowelain Mae G. Yumang, Chelsea Kate F. Jose and Danielle Camille P. Canillo
Department of Science and Technology, General Santos Avenue, Bicutan, Taguig City, 1631 Metro Manila, Philippines
*Corresponding Author: Rosalinda C. Torres, Standards and Testing Division, Industrial Technology Development Institute, Department of Science and Technology, General Santos Avenue, Bicutan, Taguig City, 1631 Metro Manila, Philippines. Email: rosalindactorres@gmail.com
Article Information
Aritcle Type: Research Article
Citation: Rosalinda C. Torres, Rowelain Mae G. Yumang, Chelsea Kate F. Jose, et al. 2019. Microencapsulation of Betalain from Philippine Beta vulgaris as stable colorant powder. Open J Food Nutri Res. 1: 17-25.
Copyright: This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Copyright © 2019; Rosalinda C. Torres
Publication history:
Received date: 28 November, 2019Accepted date: 06 December, 2019
Published date: 09 December, 2019
Abstract
Safe and non-toxic natural colorants are now gaining a lot of interest due to the negative effects of synthetic colorants to the human body and to the environment. However, processing of natural colorants is highly dependent on several parameters, thus, making it unstable. Accordingly, this study aims to produce stable beetroot colorant powder through microencapsulating betalain pigment from Beta vulgaris by spray drying technique. The red beetroots (B. vulgaris) were subjected to aqueous solid-liquid extraction. The extract was then microencapsulated using 3%w/v and 5%w/v maltodextrin DE 12 through spray-drying technology with varying parameters such as inlet temperature and feed flow rate. The physicochemical properties of the beetroot colorant were evaluated including microbial analysis, heavy metal content, and dermal irritation test. Results showed that the lowest moisture content yielded at 160°C however, a significant reduction on betalain content followed. BRB2 colorant produced at 150°C, 15mL/min with 5% MD was found to be the most favorable condition given the set of parameters. After 8 weeks, there was no significant difference on the color of each colorant indicating its stability. Moreover, the microencapsulated betalain is deemed safe as it falls under the reference limits for its toxicity evaluation.
Keywords: Microencapsulation; Betalain; Spray-drying; Beta vulgaris; Physicochemical properties; Safety assessment; Beet root; Natural colorant
Introduction
In recent days market for application of synthetic colorants has decreased in favor of natural colorants. Concerns have also been raised about the deleterious effects associated with artificial food dye [1], which has contributed to increased regulatory pressure and demand for natural colorants [2]. Synthetic colorants are more widely used due to their lower production cost and greater stability [3]. However, these have been associated with harmful effects on humans, such as potential carcinogenicity and development of attention deficit hyperactivity disorder (ADHD) in children [4]. Furthermore, there have been growing ecological concerns regarding its use because when these colorants are not fixed in the food matrix, they contribute to the industrial wastewater [5]. Interests in the use of natural colorants has continued to grow due to the stringent environmental standards imposed by many countries since they are believed to be safe, and non-toxic to humans [6]. One of the known potential sources of colorants is Beta vulgaris, commonly called as beetroot derived from Amaranthaceae family. Betalain is an aromatic indole molecule and vacuolar pigment derived from betalamic acid. It is the pigment present in the beetroot [7]. It can be divided into two structural groups, the yellow betaxanthins and red-purple betacyanins. Betanin accounts for the 75%-95% red color in betacyanins. However, the problem with natural pigments is their stability. These pigments are easily affected by temperature, light, pH, oxidation and water activity. So, in order for these pigments to be stable, microencapsulation is recommended to protect them from degradation. Microencapsulation or encapsulation is a technique wherein the bioactive compound of a material is coated with a thin film of a biopolymer in order to improve its stability, prevent its degradation, oxidation and other conditions. Several microencapsulating agents such as polysaccharides, starches, polymers were often used for encapsulation [8]. However, the most commonly used microencapsulating agent was maltodextrin due to its good compromise between cost and effectiveness, availability in different molecular weights, bland in flavor, low viscosity and hygroscopicity and excellent stability to oxidation [9]. In industries, spray-drying was the technique commonly used in producing microencapsulated dried powders from extracts as it is much lower in cost than compared to other drying techniques [10]. Thus, this research aims to produce microencapsulated beetroot colorant powder through microencapsulation of betalain pigment by spray drying technique. The microencapsulated beetroot powder imparts a soft and lustrous color which can be compared with synthetic colorants.
Materials and Methods
Materials
Fresh red beetroots (Beta vulgaris) were collected and carefully selected with complete matured state, round and large approximately 10-15cm in diameter from Atoc, Benguet, Philippines. These were washed and cleaned with 2% hypochlorite solution to remove dirt. The peels were removed, and the red flesh was subsequently sliced into small sizes. The sliced beetroot flesh was stored at ultralow freezer for further use. The microencapsulating carrier, maltodextrin (DE12) used for spray drying was purchased from Allyson’s (Araneta Avenue, Philippines).Fresh red beetroots (Beta vulgaris) were collected and carefully selected with complete matured state, round and large approximately 10-15cm in diameter from Atoc, Benguet, Philippines. These were washed and cleaned with 2% hypochlorite solution to remove dirt. The peels were removed, and the red flesh was subsequently sliced into small sizes. The sliced beetroot flesh was stored at ultralow freezer for further use. The microencapsulating carrier, maltodextrin (DE12) used for spray drying was purchased from Allyson’s (Araneta Avenue, Philippines).
Preparation of betalain extract
Betalain extraction was carried out by aqueous solid-liquid extraction technique. One thousand grams of sliced beetroots were blended with 3000mL deionized water using a waring blender and the extraction was carried out overnight under low temperature (4°C). After maceration, the solution was filtered and was set aside for microencapsulation process.
Microencapsulation of extracted betalain
The extracted solution was microencapsulated using two different maltodextrin (MD) concentrations (3% w/v and 5% w/v DE12 maltodextrin). The extract with the microencapsulating agent was mixed thoroughly until a homogenized solution was obtained. The solution was set aside for spray- drying process.
Spray drying process of betalain extract
The beetroot solution was fed into the spray dryer. The operational parameters were inlet temperatures of 150°C and 160°C, feed flow rates of 15mL/min and 20mL/min and air flow pressure of 2kg/cm3. The spray dried powder was collected in an insulated cylindrical vessel connected at the end of the cyclone and was packed in polyethylene pouch with silica gel and stored in desiccator at 25°C.
Sample Analysis
Moisture Content
Moisture content was determined using a moisture analyzer (Shimadzu MOC-120H) set at 105°C. One gram of powder was placed on the aluminum plate and was read for 10 minutes. The moisture content was recorded and done in triplicates.
Hygroscopicity
Hygroscopicity was determined according to Cai and Corke (2000) method [11] with some modifications. About 1g of powder samples were weighed and placed in a 25°C glass desiccator prepared with saturated NaCl solution (67% RH). After 1 week, the samples were weighed and hygroscopicity was expressed as a gram of adsorbed moisture (g/100g) dry solids.
Total betalain content
The total betalain content of microencapsulated beetroot powder was determined spectrophotometrically with slight modification according to Wybraniec & Mizhari [12] and Stintzing, et al. [13]. Approximately 0.2g of colorant powder was weighed and diluted in deionized water at 25 mL volumetric flask. The absorbance was scanned at spectrophotometric wavelength of 400-700nm using L7 double beam UV-VIS spectrophotometer. The absorbance was recorded at wavelength of 486nm and 536nm corresponding to betacyanin and betaxanthin, respectively. The total betalain content was calculated as:
TBC=BC+BX
A= absorbance at 486nm (betaxanthin) and 536nm (betacyanin)
MW= molecular weight; 308g/mol (betaxanthin), 550g/mol (betacyanin)
V=volume of the solution
DF= dilution factor
ε= molar extinction coefficients; 40000L/mol cm (betaxanthin), 60000L/mol cm (betacyanin)
L=path length of the cuvette (1cm)
W=weight of the microencapsulated beetroot
Color Analysis
Color analysis was measured in terms of HUNTER’S luminosity (L*), red versus green (a*) and yellow versus blue value (b*) using Lovibond LC 100 spectrocolorimeter.
Heavy Metals Analysis
Heavy metals analysis was conducted at Mach Union Water Laboratory, Inc., Las Piñas City, Philippines using the Test Reference: Method 3050B [14].
Microbial Content
The test was conducted at the Microbiology Laboratory of ITDI-Standards and Testing Division, DOST, Philippines using Test Reference: Bacteriological Analytical Manual On-line, 2001 [15]
Dynamic Light Scattering (Malvern Zetasizer)
The test was conducted at the University of the Philippines Los Baños Nanoscience and Technology Facility Analytical and Instrumentation Service Laboratory, Laguna, Philippines. Prior to analysis, the samples were suspended in HPLC grade water. Average particle size was determined using three 6 measurements at 90° angle through a Malvern Zetasizer Nanoseries Nano-ZS90.
Dermal Irritation Test
The test was conducted at the Pharmacological and Toxicological Laboratory, Standards and Testing Division, DOST-ITDI, Philippines following the standard procedure of the Organization for Economic Cooperation and Development (OECD) TG 404 (2002) method [16].
Results and Discussion
Physicochemical properties of microencapsulated betalain pigment were significantly influenced by application of various spray-drying parameters and carrier agent. Table 1 provides the resulting properties after subjecting to different inlet temperature, feed flow rate and concentration of microencapsulating carrier. BRB2 represents the microencapsulated powder at 150°C, 15 mL/min with 5% MD, BRB6 at 160°C, 15 mL/min with 5% MD, BRB5 at 150°C, 15 mL/min with only 3% MD and BRB7 at 150°C, 20 mL/min with 5% MD.
|
Table 1: Physical properties of microencapsulated betalain colorant powder. |
||||
|
Code |
BRB2 |
BRB6 |
BRB5 |
BRB7 |
|
Inlet Temperature |
150°C |
160°C |
150°C |
150°C |
|
Flow Rate |
15mL/min
|
15mL/ min |
15mL/ min |
2 20mL/min |
|
Microencapsula- ting carrier concentration |
5% 12DEMD |
5% 12DEMD |
3% 12DE MD |
5% 12DE MD |
|
Powder productivity yield |
18% |
23% |
17% |
22% |
|
Moisture Content |
5.49% |
2.78% |
7.86% |
6.86% |
|
Hygroscopicity |
5.46 |
9.74 |
8.75 |
9.32 |
|
pH |
3.45 |
3.25 |
3.20 |
3.23 |
The moisture content of the microencapsulated powder exhibits a complex behavior as it is not only affected by one specific parameter. As shown in the table above, the lowest moisture content was obtained at 160°C inlet temperature and 5% w/v maltodextrin concentration. This may be due to the fact that at higher inlet temperatures, greater evaporation capacity is acquired which leads to a larger chemical potential between atmospheric air and water during process [17]. On the other hand, it can be observed how it is also influenced by the given feed flow rates. Feed flow rate at 15 mL/min delivered a lower moisture content compared to that of 20 mL/min with an exception of BRB5 which carries the smallest concentration of maltodextrin. High feed rate according to previous research promotes higher moisture content as it directly affects water activity [18] while decreasing the amount of microencapsulating agent promotes decreased levels of total feed solids and thereby increasing the corresponding level of moisture bound for evaporation [19,20]. In relation, the hygroscopicity which is the capacity of the powder to absorb ambient moisture is also affected by increasing inlet temperature. Hygroscopicity and moisture content in this manner is indirectly proportional as greater moisture gradient is favored at low moisture content [21].
Moreover, the microencapsulation yields range from 17%-23%. Higher powder recovery was found with increasing concentration of maltodextrin and inlet temperature. It was also seen that the powder recovery loss may be associated with the uncollected powder that deposited on the chamber wall of the spray dryer.
From the different parameters used in microencapsulating the betalain, it was found out that BRB5 having a constant feed flow rate of 15 mL/min, 3% maltodextrin, and inlet temperature of 150°C has the highest betalain content of 4.67mg/L. The lower temperature of 150°C is preferred than the higher temperature of 160°C because during thermal processing of betalain rich plants, discoloration or browning is usually observed. This may due to degradation reactions such as isomerization, decarboxylation, and cleavage caused by heat subjection [22]. This is supported by the study of Herbach, K.M. et al. [23] wherein changes in color of pitaya pigments and betacyanin content are found to cause pigment reconstitution which greatly affects the physical color of the pigment when heated. Moreover, according to the study of Janiszewska, E. & Wlodarczyk, J. [24] and Finney J., Buffo R., Reineccius G.A. [25] an increase in the inlet temperatures causes a decrease in the particle density. Thus, the use of lower inlet temperature of 150°C is favored to prevent betalain degradation and to yield higher particle density.
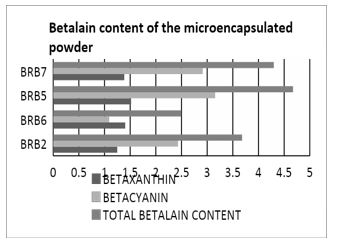
Figure 1: Total betalain content of microencapsulated powder.
The concentration of heavy metals of the betalain colorant powder was determined. The test result showed that the concentration of arsenic, lead and mercury fall under the allowable limit set by ASEAN Guidelines for commercially available colorants. This indicates that the betalain powder colorant has low concentration of heavy metals and does not impose high risk of hazard on health.
|
Table 2: Heavy Metal Content of microencapsulated Betalain. |
||
|
|
RESULTS |
ASEAN |
|
Arsenic, ppm |
<0.007 |
<3ppm |
|
Lead, ppm |
<0.002 |
<20ppm |
|
Mercury, ppm |
<0.05 |
<1ppm |
In reference to the Bacteriological Analytical Manual (2001), the microencapsulated betalain was tested for the presence of molds and yeast, total coliform, Staphylococcus aureus, Salmonalla sp., respectively indicating <100, <10, <10, and absent. This signifies its safety from common microorganisms.
|
Table 3: Microbial Profile of Microencapsulated Betalain. |
|
|
|
RESULTS |
|
Molds and Yeast Count (CFU/g sample) in agar plate, 30C, 5-7 days incubation |
<100 |
|
Total Coliform Count (CFU/g sample) in agar plate, 35C, 48 hrs incubation |
<10 |
|
Staphylococcus aureus (CFU/g of sample) in agar plate, 35C, 48 hrs incubation |
<10 |
|
Salmonella sp. Detection-Presumptive Test (per 25g of sample) |
Absent |
The Hunter’s Lab color values of the powder was measured initially and after 8 weeks. This allows the determination of the stability of the colorant over time. The “L” value indicates the lightness or darkness of the pigment. Meanwhile, color value “a” indicates the redness (+) and greenness (-) of the powder. Lastly, “b” values indicate blueness (-) or yellowness (+) of the powder. The “L” values of all the powders decreased after 8 weeks so as the “a” and “b” values. However, when statistical analysis was done, it shows no significant difference between the initial and after 8 weeks color values (L, a & b) of the powder, indicating stability of the pigment.
|
Table 4A: Stability of color after 8 weeks of study of BRB2 and BRB6. |
||||
|
|
BRB2 |
BRB2 |
BRB6 |
BRB6 |
|
|
I Initial Color Values |
Color values after 8 weeks |
I Initial Color Values |
Color Values after 8 weeks |
|
L |
3 8.8 |
34.8 |
39.6 |
32.5 |
|
a |
3 8.8 |
38.4 |
39.6 |
37.0 |
|
b |
- 4.9 |
- 3.0 |
- 8.8 |
- 7.3 |
|
Table 4B: Stability of color after 8 weeks of study of BRB5 and BRB7. |
||||
|
|
BRB5 |
BRB5 |
BRB7 |
BRB7 |
|
|
Initial Color Values |
Color Values after 8 weeks |
Initial Color Values |
Color Values after 8 weeks |
|
L |
35.1 |
25.7 |
39.6 |
36.0 |
|
a |
43.1 |
36.4 |
38.7 |
42.2 |
|
b |
- 11.3 |
- 7.3 |
- 9.0 |
- 8.9 |
The particle size distribution of the microencapsulated betalain was determined through Dynamic Light Scattering. This indicates the size of the powder in diameter nanometers of the given sample shown in intensity.
Figure 2 shows the graph of the particle size distribution of the microencapsulated betalain with 5% maltodextrin. As observed, most size of the powder ranges from 500-1000d.nm.
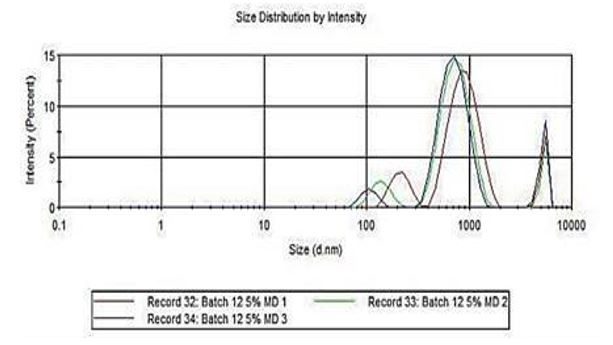
Figure 2: Size Distribution Intensity using Malvern Zetasizer ver 7.02.
|
Table 5: Dynamic Light Scattering (Particle Size) Analysis using Malvern Zetasizer ver 7.02. |
||
|
|
33% maltodextrin beetroot colorant |
55% maltodextrin beetroot colorant |
|
|
Z-Average (d. nm) |
Z-Average (d. nm) |
|
Control 1 |
517.3 |
8 06.8 |
|
Control 2 |
548.2 |
773.7 |
|
Control 3 |
442.5 |
813.8 |
From the Dynamic Light Scattering results, the colorant with 5% maltodextrin has relatively bigger size in diameter nanometers as compared to the 3% maltodextrin. According to Tontul and Topuz [26], the size of the particles is directly related to the viscosity of the spray drying feed solution. Thus, it is expected that microencapsulation with 5% maltodextrin would yield larger particles.
|
Table 6A: Dermal Irritation Test. |
||||||
|
Rabbit number |
Scoring of skin reaction Erythema & eschar formation |
|||||
|
|
4 hrs |
24 hrs |
2 days |
3 days |
7 days |
14 days |
|
1 |
0 |
0 |
0 |
0 |
0 |
0 |
|
2 |
0 |
0 |
1 |
0 |
0 |
0 |
|
3 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Table 6B: Dermal Irritation Test. |
|||||||
|
Rabbit number |
Scoring of skin reaction Edema formation |
||||||
|
|
4 hrs |
24 hrs |
2 days |
3 days |
7 days |
14 days |
|
|
1 |
0 |
0 |
0 |
0 |
0 |
0 |
|
|
2 |
0 |
0 |
0 |
0 |
0 |
0 |
|
|
3 |
0 |
0 |
0 |
0 |
0 |
0 |
|
The scoring values for the dermal irritation test are as follows: 0- no edema/ erythema, 1- very slight edema/erythema, 2- slight edema/erythema, 3- moderate edema/erythema, 4- severe edema/erythema. By looking at Table 6A and 6B, almost all results showed 0 indicating the absence of edema or erythema making it safe for topical application.
Conclusion
In this study, the microencapsulated betalain colorant from beetroot was produced with the use of the spray-drying technique. It was found out that the physical and chemical properties of microencapsulated betalain powder are affected by the concentration of the microencapsulating agent, the inlet temperature and feed flow rate during spray drying. Increase in inlet temperature leads to lower moisture content and higher powder yield. However, it increases the hygroscopicity value and causes the pigment degradation resulting in lower betalain content. The same goes with increasing the feed flow rate except that the moisture content is higher at this condition. Meanwhile, higher concentration of maltodextrin produced higher powder yield and lower moisture content. Degradation of the color of beetroot betalain powder was likewise protected by microencapsulation through spray drying. The microencapsulated beetroot colorant was found to be safe with test results of the microbial profile, heavy metal content, and dermal irritation test fall within the reference limit set for colorant powders. Future research of natural colorant from beetroot is likely to focus on the stability of the colorants and good microencapsulation efficiency. It can be envisioned then that with a deep understanding of microencapsulated natural colorants and improvement in its production, these can be used as an alternative to synthetic dyes.
Acknowledgement
The authors acknowledge the Philippine Council for Industry, Energy and Emerging Technology Research and Development (PCIEERD) for the financial support.
References
1. Amchova P, Kotolova H, Ruda-Kucerova J. 2015. Health safety issues of synthetic food colorants. Regulatory Toxicology and Pharmacology. 73: 914-922. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/26404013
2. Shadid M, Islam S, Mohammad F. 2013. Recent advances in natural dye applications: A review. Journal of Cleaner Production.53: 310-331. Ref.: http://tiny.cc/md49gz
3. Potera C. 2010. The artificial food dye blues. Environ Health Perspect. 118: 428. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/20884387
4. Feng J, Cerniglia CE, Chen H. 2012. Toxicological significance of azo dye metabolism by human intestinal microbiota. Front. Biosci. 4: 568-586. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/22201895
5. Dotto GL, Pinto LA, Hachicha MA, et al. 2015. New physicochemical interpretations for the adsorption of food dyes on chitosan films using statistical physics treatment. Food Chemistry. 171: 1-7. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/25308634
6. Rymbal H, Sharma RR, Manish Srivastav. 2011. Biocolorants and its implications in health and food industry-a review. International Journal of Pharma Research. 3: 2228-2244. Ref.: http://tiny.cc/dy49gz
7. Ravichandran K, Saw NMMT, Mohdaly AAA, et al. 2013. Impact of processing beet root on betalain content and antioxidant activity. Food Research International. 50: 670-675. Ref.: http://tiny.cc/1549gz
8. Jeyakumari A, Zynudheen AA, Parvathy U. 2016. Microencapsulation of bioactive food ingredients and controlled release-a review. MOJ Food Process Technology. 2: 214-224. Ref.: https://bit.ly/2s0lvs0
9. Madene A, Jacquot M, Scher J, et al. 2006. Flavour encapsulation and controlled release-a review. International and Journal of Food Science and Technology. 41: 1-21. Ref.: https://bit.ly/2Lnov91
10. Fang Z, Bhandari B. 2011. Effect of spray drying and storage on the stability of bayberry polyphenols. Food Chemistry. 129: 1139-1147. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/25212349
11. Cai YZ, Corke H. 2000. Production and Properties of Spray dried Amaranthus Betacyanin Pigments. Journal of Food Science. 65:1248-1252. Ref.: https://bit.ly/2qlwig3
12. Wybraniec S, Mizrahi Y. 2002. Fruit Flesh Betacyanin Pigments in Hylocereus Cacti. Journal of Agricultural and Food Chemistry. 50: 6086-6089. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/12358484
13. Stintzing FC, Herbach KM, Mosshammer MR, et al. 2005. Color, betalain pattern and antioxidant properties of cactus pear (Opuntia spp.) clones. Journal of Agricultural and Food Chemistry. 53: 442-451. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/15656686
14. Method 3050B. Acid Digestion of Sediments, Sludges and Soils; SW-846 Test Method for Evaluating Solid Waste: Physical/Chemical Methods
15. Feng P. 2001. Appendix 1- Rapid Methods for detecting foodborne pathogens. Bacteriological Analytical Manual Online.
16. Organization for Economic Cooperation and Development 2002. Test No. 404: Acute Dermal Irritation/Corrosion, OECD Publishing, Paris. doi.org/10.1787/9789264070622-en
17. Chegini GR, Ghobadian Barat. 2005. Effect of Spray-Drying Conditions on Physical Properties of Orange Juice Powder. Drying Technology - DRY TECHNOL. 23: 657-668. Ref.: https://bit.ly/2Pf2pqd
18. Mishra P, Brahma A, Seth D. 2017. Physicochemical, functionality and storage stability of hog plum (Spondia pinnata) juice powder produced by spray drying. Journal of food science and technology. 54: 1052-1061. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/28416854
19. Grabowski JA, Truong VD, Daubert CR. 2006. Spray drying of amylase hydrolyzed sweet potato puree and physiochemical properties of powder. J Food Sci. 71: 209-217. Ref.: https://bit.ly/34NF8Ci
20. Kha CT, Nguyen HM, Roach DP. 2010. Effects of spray drying conditions on the physicochemical and antioxidant properties of the Gac (Momordica cochinchinensis) fruit aril powder. J Food Eng. 98: 385-392. Ref.: https://bit.ly/2Yk0epF
21. Tonon RV, Brabet C, Hubinger MD. 2008. Influence of process conditions on the physicochemical properties of açai (Euterpeoleraceae Mart.) powder produced by spray drying. J Food Eng. 88: 411-418. Ref.: https://bit.ly/33Saq9Z
22. Azeredo H. 2009. Betalain: properties, sources, applications, and stability - a review. International Journal of Food Science and Technology. 44: 2365-2376. Ref.: https://bit.ly/38aAK2b
23. Herbach KM, Stintzing FC, Carle R. 2004. Thermal degradation of betacyanins in juices from purple pitaya [Hylocereus polyrhizus (Weber) Britton & Rose] monitored by high-performance liquid chromatography–tandem mass spectrometric analyses. European Food Research and Technology. 219: 377-385. Ref.: https://bit.ly/2rTb2im
24. Janiszewska E, Wlodarczyk J. 2013. Influence of spray drying conditions on beetroot pigments retention after microencapsulation process. Acta Agrophysica. 20: 343-356. Ref.: https://bit.ly/2LpLO1K
25. Finney J, Buffo R, Reineccius GA. 2002. Effects of type of atomization and processing temperatures on the physical properties and stability of spray-dried flavors. J. Food Sci. 67: 1108-1114. Ref.: https://bit.ly/2LosTo8
26. Tontul I, Topuz A. 2017. Spray-drying of fruit and vegetable juices: Effect of drying conditions on the product yield and physical properties. Trends in Food Science & Technology. 63: 91-102. Ref.: https://bit.ly/2rSVkDV




















