Indexing & Abstracting
Full Text
Research ArticleDOI Number : 10.36811/ijpmh.2019.110001Article Views : 3141Article Downloads : 51
Fast-acting effects of l-tetrahydropalmatine on depression and anxiety in mice
Rui Li1, Wai-Kin Mat1, Wing-Man Chan2, T Yiu-Cheong Ho1, Rigil K Yeung1, Chi-Him Poon1, Lawrence W-Y Wong2, Ian D Williams2, Mingqi Qiao3 and Hong Xue1,3*
1Division of Life Science, State Key Laboratory of Molecular Neuroscience and Applied Genomics Center, Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong, P.R. China
2Department of Chemistry, Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong, P.R. China
3School of Basic Medical Sciences, Shandong University of Traditional Chinese Medicine, Jinan, Shandong 250355, P.R. China
*Corresponding author: Professor Hong Xue, Division of Life Science, Hong Kong University of Science and Technology, Clear Water Bay, Hong Kong, China, Tel: +852 23588707; Fax: +852 23581552; Email: hxue@ust.hk
Article Information
Aritcle Type: Research Article
Citation: Rui L, Wai-Kin M, Wing-Man C, et al. 2019. Fast-acting effects of l-tetrahydropalmatine on depression and anxiety in mice. Int J Psychiatr Ment Health. 1: 01-12.
Copyright:This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Copyright © 2019; Rui L
Publication history:
Received date: 08 January, 2019Accepted date: 21 January, 2019
Published date: 22 January, 2019
Abstract: The racemate dl-tetrahydropalmatine (dl-THP) is known for its analgesic and sedative effects, and has been shown by us to be a potential agent for the treatment of anxiety.Herein, to delineate the therapeutic potentials of its different isomeric forms, the behavioral effects of l-THP, dl-THP and d-THP were compared regarding their anxiolytic and antidepressant properties in mouse behavioral models using the elevated plus-maze test and tail suspension test respectively. The anxiolytic and antidepressant effects of both l-THP and dl-THP were evident in forty-five minutes following oral administration. Moreover, l-THP exhibited much greater anxiolytic potency in the elevated plus-maze (0.1-2.5 mg/kg) and antidepressant potency in the tail suspension test (0.5-5.0 mg/kg) than dl-THP, whereas d-THP was inactive in either of these tests. As well, l-THP enhanced sociability and preference for social novelty at 0.1-0.5 mg/kg in Crawley’s three-chamber behavioral tests, and inhibited the amphetamine-induced manic-like hyperactivity of amphetamine-sensitized mice at 0.05-0.2 mg/kg. These pharmacological actions of l-THP were unaccompanied by any significant locomotor or myorelaxant side-effects. Co-administration of flumazenil, a GABAA receptor antagonist, inhibited the anxiolytic and antidepressant effects of l-THP, even though the binding affinity of l-THP was higher for dopamine D2-like receptors than for GABAA receptors. On this basis, l-THP displayed potential as a fast-acting drug for the treatment of anxiety, depression and bipolar disorder.
Keywords: l-THP; dl-THP; Anxiolysis; Antidepressant; GABAA receptor; Fast-acting
Introduction
The racemate compound dl-tetrahydropalmatine (dl-THP) is a major constituent of the medicinal herb Corydalis yanhusuo W.T. Wang. Pharmacologically, dl-THP is known to display anti-epileptic, anti-hypertensive, as well as antinociceptive and sedative-tranquilizing activities[1-3].For both the antinociceptive and sedative-tranquilizing effects, its l-THP constituent was the effective component; the d-THP enantiomer exerted little effect by itself, but enhanced the antinociceptive effect of l-THP[3]. Administration of l-THP also provided effective treatment for addictions to oxycodone, cocaine and methamphetamine [4-6], and for posttraumatic stress disorder [7].
Previously, we found that acute administration of dl-THP could induce anxiolysis in mice based on the elevated plus-maze test [8], and this was also found to be the case with rats [9]. The anxiolytic effect was blocked in mice, and partially blocked in rats, by the GABAA receptor benzodiazepine (BZ) site antagonist flumazenil at 1.25 mg/kg. In the present study, the potential effects of l-THP, d-THP and dl-THP for treatment of anxiety and depression were assessed in mice using the elevated plus-maze test and tail suspension test respectively. As well, the effects of l-THP on sociability and preference for social novelty were examined using the Crawley’s three-chamber behavioral tests, and its effects on amphetamine-induced hyperactivity examined based on locomotor activity.
Body
Methodology
Chemicals
The l-THP was obtained from Santa Cruz Biotechnology (CA, USA), and dl-THP from Indofine Chemical Company (98% purity; NJ, USA); d-THP was prepared as described [10]. Diazepam, flumazenil, D-amphetamine sulfate and γ?aminobutyric acid (GABA) were obtained from Sigma Chemical (St. Louis, USA). [3H]-flunitrazepam ([N-methyl-3H], 88.0 Ci/mmol) and [3H]-spiperone ([benzene ring-3H], 15.7 Ci/mmol) were obtained from PerkinElmer Life Sciences (Boston, USA). The dl-THP, d-THP and l-THP were dissolved in 0.9% NaCl in the presence of 1% H2SO4 and adjusted to pH 7.4 with NaHCO3. Diazepam was dissolved in 0.9% NaCl in the presence of 1% DMSO for animal tests. GABA was dissolved in 50 mM Tris-Cl, and D-amphetamine sulfate (corrected for weight of sulfate) was dissolved in 0.9% NaCl.
Animals
Male ICR mice (20 - 35 g) were housed in groups of five with food and water ad lib,and kept on a 08:00 hour to 20:00 hour light cycle. Drugs (10 ml/kg) were administered to mice. All animal experiments were pre-approved by the HKUST Animal Ethics Committee and conducted in accordance with the Code of Practice for Care and Use of Animals for Experimental Purposes of the Agriculture, Fisheries and Conservation Department and the Department of Health of Hong Kong Special Administrative Region. All procedures were carried out in a quiet, air-conditioned laboratory between 08:00 h and 13:00 h at ambient temperature of 20-22 ºC.
Elevated plus-maze test
Male ICR mice 4 to 6 weeks old were randomly separated into groups. Vehicle(0.9% NaCl. p.o.), 0.01 - 2.5 mg/kg l-THP p.o., 0.1 - 2.5 mg/kg dl-THP p.o., 1.0 - 5.0 mg/kg d-THP p.o., or 1 or 3 mg/kg diazepam i.p. was administered 45 minutes prior to the test, which was performed as described[11]. The increased number of open arms entries and percentage of time spent in open arms during a 5-minute test period signified an anxiolytic effect [12,13].
To test whether the anxiolytic effect elicited by l-THP could be blocked by the benzodiazepine (BZ) site antagonist flumazenil, male 4-6-week-old mice were randomly separated into three groups. The first group received vehicle (0.9 % NaCl) p.o. 45 minutes before test and 1.25 mg/kg flumazenil i.p. 15 minutes before test; the second group received either 1.0 mg/kg l-THP or p.o. 45 minutes before test, and vehicle i.p. 15 minutes before test; and the third group received 1.0 mg/kg l-THP p.o. 45 minutes before test and 1.25 mg/kg flumazenil i.p. 15 minutes before test.
Tail suspension test
Male ICR mice 4 to 6 weeks old were randomly separated into groups, and orally administered with vehicle (0.9 % NaCl), l-THP(0.2-5.0 mg/kg p.o.), or dl-THPord-THP (1.0-5.0 mg/kg p.o.) 45 minutes prior to testing, or 30 mg/kg imipramine p.o. 30 minutes prior to testing. In the test, mice were taped by the tail to a bar and suspended 15 cm above ground for 6 minutes. The total time of immobility was scored manually from 2 min to the end of the 6-minute test period.
To test whether the antidepressant effect elicited by dl-THP and l-THP could be blocked by flumazenil, male 4-6-week-old mice were randomly separated into three groups. The first group received vehicle (0.9 % NaCl) p.o. 45 minutes before test and 1.25 mg/kg flumazenil i.p. 15 minutes before test; the second group received 1.0 mg/kg l-THP or 2.0 mg/kg dl-THP p.o. 45 minutes before test, and vehicle i.p. 15 minutes before test; and the third group received 1.0 mg/kg l-THP or 2.0 mg/kg dl-THP p.o. 45 minutes before test and 1.25 mg/kg flumazenil i.p. 15 minutes before test.
Crawley’s three-chamber behavioral tests
Female ICR mice 8 to 10 weeks old were randomly separated into groups, and orally administered with vehicle (0.9 % NaCl p.o.) or l-THP (0.1 or 0.5 mg/kg p.o.)45 minutes prior to testing. The test apparatus (19 x 45 cm) comprised a rectangular plexiglass three-chamber box with a middle chamber and two side chambers. Each of the two side chambers housed a wire-cup container with removable lid that was large enough to hold a single mouse, and allowed air exchange between the interior and exterior of the container but too small to enable physical contact between an animal inside and one on the outside.
As described by Kaidanovich-Beilin et al. [14], at the start of test, a test mouse was placed at the center of the middle chamber for adaptation. After 5 minutes, a 10-minute test session-1 was started by placement of a stranger-1 mouse inside the wire-cup in one of the side chambers. The number and durations of interactions made by test mouse with the wire-cup housing, and the wire-cup not housing, stranger-1 mouse were recorded separately. Upon completion of test session-1, a 10-minute test session-2 was started by placement of a stranger-2 mouse in the wire-cup that was left empty in session-1. The number and duration of interactions made by test mouse with either stranger-1 or stranger-2 mouse were again recorded. In session-1, the percentage of total number or percentage of duration displayed by the test mouse interacting with stranger-1 mouse was estimated as 100% x [number or duration of interaction with stranger-1 mouse]/[total number or duration of interaction with either strange-1 mouse or with empty cup].In session-2, the percentage of total number or percentage of duration displayed by the test mouse interacting with stranger-2 mouse was estimated as 100% x [number or duration of interaction with stranger-2 mouse]/[total number or duration of interaction with either strange-2 mouse or with stranger-1 mouse].Thereby session-1 enabled an estimation of the sociability of the test mouse, whereas session-2 enabled an estimation of the preference of the test mouse for social novelty.
Locomotor activity test
Male ICR mice 4 to 6 weeks old were randomly separated into groups. Vehicle (0.9% NaCl, p.o.), or 0.01 - 2.5 mg/kg p.o.l-THPor dl-THP, or 1 or 3 mg/kg i.p. diazepam was administered 45 minutes before the test.The ZIL-2 apparatus (Beijing Institute of Materia Medica, 60 cm x 60 cm x 12 cm) employed for the test consisted of four plastic cylindrical containers, each equipped with six evenly spaced infrared beam-photocell monitors. The number of transitions across the beams was recorded over a period of 5 minutes, yielding an estimate of the level of locomotor activity.
Manic-like behavior
Manic-like behavior was assessed based on the method of Pathak et al.[15]: 6 to 7-week-old male ICR mice were given daily injections for 5 days of vehicle (Veh, 0.9% NaCl) in the Veh group, or 1.8 mg/kg D-amphetamine in the amphetamine-only (Amp) group and in the l-THP treatment groups. This was followed by withdrawal from all groups the vehicle or D-amphetamine injections for 7 days to induce sensitization toward D-amphetamine in the Amp group and l-THP treatment groups. At 30 minutes prior to locomotor activity testing with the ZIL-2 apparatus in order to assess hyperactivity as an indication of manic-like behavior, the Veh group and Amp group were administered with Veh p.o., and the l-THP treatment groups were administered with 0.05 - 0.2 mg/kg l-THP p.o.. At 10 minutes prior to testing, the Veh group received vehicle i.p., and both the Amp group and the l-THP treatment groups received 0.9 mg/kg i.p. amphetamine.
Horizontal wire test
Male ICR mice 4 to 6 weeks old were randomly separated into groups. Vehicle (0.9 % NaCl p.o.), 0.5-30.0 mg/kg p.o.l-THP or dl-THP, or 1 or 3 mg/kg diazepam i.p. was administered 45 minutes prior to the test. Each mouse was lifted by the tail and allowed to grasp on to a horizontally strung wire (1 mm diameter, 20 cm long and 20 cm above the table) with its forepaws and released. For each animal, the number of falls made within 60 seconds was recorded. Increased number of falls provided an indication of muscle relaxation[8].
Radioligand binding assays
Synaptosomal membranes prepared from male Sprague-Dawley rat cerebral cortex [16],were employed for radioligand binding assays using the GABAA receptor BZ-site ligand [3H]-flunitrazepam or dopamine D2-like receptor ligand [3H]-spiperone as previously described [11]. Half-maximal inhibition by l-THP or dl-THP (IC50) was determined by non-linear regression using Prism 5.0 (GraphPad Software). Ki values were calculated from Ki=IC50/ [1+([3H]/Kd)], where [3H] was the concentration of [3H]-ligand, and Kd the dissociation constant of the [3H]-ligand. The Kd for [3H]-flunitrazepam was derived from Huen et al. [17], and that for [3H]-spiperone from Chatterjee et al. [18].
HPLC analysis
The HPLC system employed in this study consisted an Agilent system with G1311B pump, G1329B sample injector and G4212B detector, and a Lab Advisor Software (Agilent, Santa Clara CA, USA). The column used was a CHIRALPAK®AD-H column (250mm×4.6mm ID, 5µm, Daicel Chiral Technologies, Shanghai, China), with hexane-isopropanol (2:8, v/v) as mobile phase, flow-rate of 1.0 ml min−1, and monitoring of effluent at 210 nm. Peak identification was performed based on retention time and comparison of the UV spectrum of eluted compounds against standard spectra. The ratio between d- and l-THP was determined by comparison of the areas under the elution curve.
Data analysis
Results were expressed as mean±standard error of the mean (S.E.M.). One-way ANOVA test was performed on behavioral data and, where significance was reached (p<0.05), multi-group comparisons were made using theNewman-Keuls’ test.
Results
Anxiolytic effect of l-THP
In the elevated plus-maze test for anxiety, ICR mice treated with oral l-THP displayed dose-dependent anxiolysis up to p<0.001at doses of 0.1-2.5 mg/kg based on increased time spent in the open arms, peaking at 0.50 mg/kg with 40.2±4.65 % time spent in open arms compared to 14.1±1.47 % time spent by controls. Diazepam (DZ), a BZ site agonist employed as positive control, induced significant anxiolytic effect (p<0.001) at 1 mg/kg and 3 mg/kg with 46.0±5.53% and 75.1±5.24 % time spent in the open arms respectively (Figure 1A). Co-administration of flumazenil (1.25 mg/kg, i.p.), a BZ-site antagonist, completely blocked the anxiolytic effect induced by 1 mg/kg l-THP (Figure 2A). dl-THP displayed dose-dependent anxiolysis at 1.0-2.0 mg/kg, peaking at 2.0 mg/kg with 45.8±5.36% time spent in open arms compared to 14.1±1.47 % time spent by controls(Figure 1B), whereas there was no anxiolysis by d-THP up to 5.0 mg/kg (Figure 1C).
Antidepressant effect of l-THP
In the tail suspension test for depression, mice treated with oral l-THP displayed an antidepressant effect at 0.5-5.0 mg/kg based on immobility time. The effect began at 0.5 mg/kgl-THP (p<0.01), and peaked at 2.0 mg/kg yielding an immobility time of 29.6±5.31 s compared to 78.9±5.85 s for the controls (p<0.001) (Figure 3A). Imipramine (Imi), a tricyclic antidepressant drug,exhibited significant antidepressant effect (p<0.001) at 30 mg/kgp.o. with an immobility time of 26.7±6.00 s (Figure 3A). The dl-THP displayed antidepressant effects at 2.0-5.0 mg/kg (Figure 3B), but not at 1.0 mg/kg, whereas there was no antidepressant effect by d-THP up to 5.0 mg/kg (Figure 3C). The antidepressant effect of 0.5 mg/kg l-THP, yielding an immobility time of 39.5±9.23 s was comparable to the antidepressant effect of 5.0 mg/kg dl-THP yielding an immobility time of 44.0±7.17s (Figure 3A,B). Co-administration of flumazenil antagonized the antidepressant effects of both l-THP and dl-THP (Figure 2B).
Enhancement of social behavior
In Crawley’s three-chamber behavioral tests, 0.1 mg/kg l-THP enhanced sociability as indicated by significant increases (p<0.05) in the percentile number of visits and duration displayed by the test mice with stranger-1 mouse (Figure 4B). The same dosage of l-THP also enhanced preference for social novelty as indicated by significant increases (p<0.05) in the percentile number of visits and duration displayed by the test mice with stranger-2 mouse (Figure 4B). In comparison, 0.5 mg/kg l-THP only enhanced sociability in terms of a significant increase (p<0.05) in the percentile number of visits by the test mice with stranger-1 mouse in the test (Figure 4A,B).
Reduction of manic-like behavior of amphetamine-sensitized mice
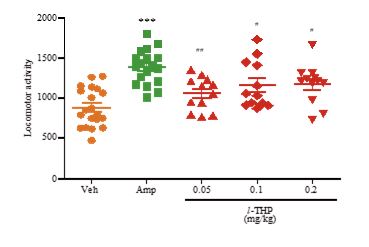
In the locomotor activity test, amphetamine-sensitized mice displayed significant hyper-locomotor activity relative to Veh treated-mice (p<0.001). Oral treatment of the amphetamine-sensitized mice with 0.05 mg/kg l-THP induced a decrease in the hyper-locomotor activity to p<0.01, and 0.1 or 0.2 mg/kg l-THP also decreased the hyper-locomotor activity to p<0.05 (Figure 5).
Figure 1: Anxiolytic effect of l-THP measured using the elevated plus-maze test. Percentage of time spent in open arms during a 5-minute period by mice treated with oral l-THP (Part A), oral dl-THP (Part B), d-THP (Part C), diazepam (DZ) or vehicle (Veh, 0.9% NaCl) is shown in red, blue, purple, green or orange respectively. n=12-25 mice in each treatment group. Data are expressed as mean ±S.E.M.; **p<0.01 and*** p<0.001 indicate significant difference between Veh group and treated groups based on Newman-Keuls’ test after one-way ANOVA.
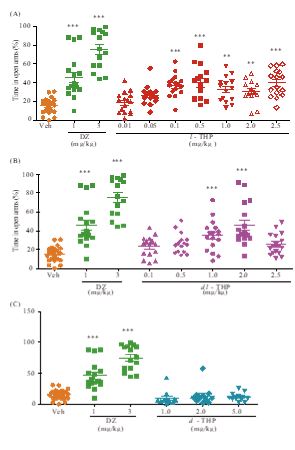
Figure 2: Anxiolytic and antidepressant effects of l-THP upon co-administration of flumazenil. (A) Time spent in open arms during a test period of 5 minutes by mice treated with flumazenil (Flu, 1.25 mg/kg, i.p.), oral l-THP (1.0 mg/kg), or oral l-THP (1.0 mg/kg) plus flumazenil (1.25 mg/kg, i.p.) is shown in orange, red or green respectively. (B) Immobility time during a test period of 4 minutes displayed by mice treated with flumazenil (1.25 mg/kg, i.p.), oral l-THP (1.0 mg/kg) or oral dl-THP (2.0 mg/kg), or oral l-THP (1.0 mg/kg) is shown in orange, red, blue or green respectively, whereas that for mice treated with oral dl-THP (2.0 mg/kg) plus flumazenil (1.25 mg/kg, i.p.) is shown in grey.n=12-20 mice per group in either Part A or Part B. Data are expressed as mean ± S.E.M; *p<0.05, **p<0.01 and ***p<0.001 indicate significant difference between the groups connected by line based on Newman-Keuls test after one-way ANOVA.
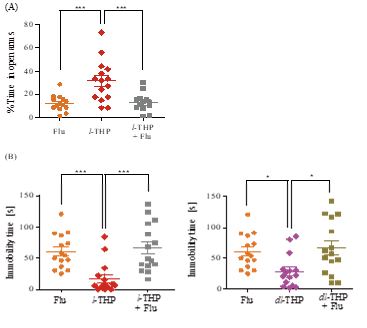
Figure 3: Antidepressant effect of l-THP measured using the tail suspension test. Immobility time during a test period of 4 minutes displayed by mice treated with indicated oral doses of l-THP (Part A), dl-THP or d-THP (Parts B and C), imipramine (Imi) or vehicle (Veh, 0.9% NaCl) is shown in red, blue, purple, green and orangerespectively. n=12-20 mice in each treatment group. Data are expressed as mean ± S.E.M.; *p<0.05, **p<0.01 and ***p<0.001 indicate significant difference between Veh group and treated groups based on Newman-Keuls’ test after one-way ANOVA.
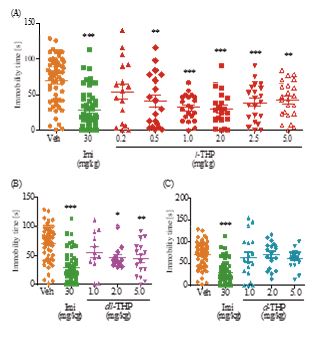
Figure 4: Effects of l-THP on social behavior. Percentages of visits and duration displayed by the test mice interacting with (A) stranger-1 mouse in the sociability test session-1, and with (B) stranger-2 mouse in the preference for social novelty test session-2 are shown for test mice treated with Veh (orange), or with oral l-THP (red). Data are expressed as mean ±S.E.M.; *p<0.05 indicates significant difference between Veh group and l-THP treated group based on Newman-Keuls’ test after one-way ANOVA. n=12-20 mice per group in both Part A and Part B.
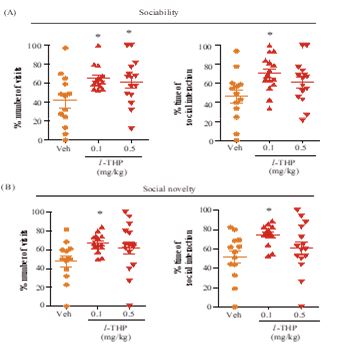
Figure 5: Effects of l-THP on manic-like behavior induced by amphetamine-sensitization. Locomotor activity was measured based on number of transitions across infrared beams. Data are expressed as mean ±S.E.M. ***p<0.001 indicates significant difference between amphetamine-sensitized (Amp) group (green) and vehicle (Veh, 0.9% NaCl) group (orange), whereas #p<0.05 and ##p<0.01 indicate significant difference between Amp group (green) and l-THP treated group (red) based on Newman-Keuls’ test after one-way ANOVA.
Locomotor activity and myorelaxant effect
Oral treatment with up to 2.5 mg/kg l-THP or dl-THP did not bring about any significant alteration in the recorded locomotor activity in the infrared-beam test. In contrast, DZ at 3 mg/kg induced a significant decrease (p<0.05) in locomotor activity. In the horizontal wire test, no myorelaxant effect was induced by up to 10 mg/kg l-THP or dl-THP. However, increased numbers of falls were observed at 30 mg/kg of l-THP or dl-THP (p<0.01), or 3 mg/kg DZ (p<0.05) (Figure 6A,B).
Figure 6: Lack of side effects of l-THP and dl-THP. (A) Locomotor activity test. Locomotor activity level of mice treated with oral l-THP (0.01-2.5 mg/kg), DZ (1 or 3 mg/kg) or vehicle (Veh, 0.9% NaCl). (B) Horizontal wire test. Number of falls by mice treated with oral l-THP (0.01 - 30 mg/kg), DZ (3 mg/kg,) or vehicle (Veh, 0.9% NaCl). Data are expressed as mean±S.E.M.; *p<0.05, **p<0.01 indicate significant difference between Veh group (orange) and treatment by l-THP (red), dl-THP (blue) or DZ (green).n= 12-20 mice per group in both Part A and Part B.

Inhibition of radioligand binding to membrane receptors
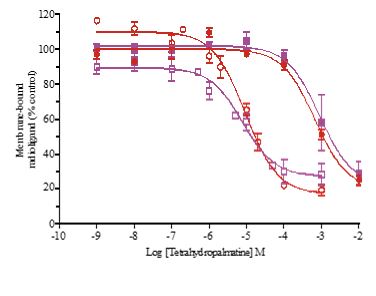
Presence of l-THP inhibited the binding of the dopamine D2-like receptor ligand [3H]-spiperone to rat cerebral cortex membranes with Ki of 5.10μM, and inhibited the binding of GABAA receptor BZ-site ligand [3H]-flunitrazepam with Ki of 368 μM. Similarly, dl-THP inhibited the binding of [3H]-spiperone with Ki of 4.27 μM, and inhibited the binding of [3H]-flunitrazepam with Ki of 494 μM (Figure 7).
Figure 7: Inhibition of [3H]-spiperone or [3H]-flunitrazepam binding to rat cerebral cortex membranes byl-THP and dl-THP. Inhibition of [3H]-spiperone binding (solid lines) by l-THP (red solid circle) or dl-THP (blue solid circle) yielded half-inhibitory Ki of 5.10±1.17 µM or 4.27±1.41 µM respectively. Inhibition of [3H]-flunitrazepam binding(dashed lines) by l-THP (red open circle)or dl-THP (blue open circle) yielded half-inhibitory Ki of 367±1.30 µM or 493±1.42 µM respectively. Each data point represents mean ±S.E.M. of three independent experiments each performed in duplicates.
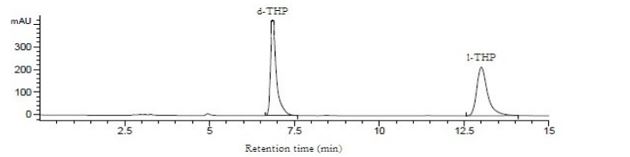
HPLC analysis of dl-THP
As indicated in Supplementary Figure 8, HPLC analysis of the dl-THP preparation employed yielded 51.0% d-THP and 49.0% l-THP.
Discussion
In the present study, acute oral administration of l-THP was found to elicit anxiolytic (at 0.1-2.5 mg/kg) and antidepressant (at 0.5-5.0 mg/kg) effects. Oral administration of l-THP also decreased the hyper-locomotor activity of amphetamine-sensitized mice, indicating the presence of an anti-manic-like effect (Figure 5).In Crawley’s three-chamber behavioral tests, 0.1 and 0.5 mg/kg oral l-THP significantly enhanced the sociability of mice, and 0.1 mg/kg oral l-THP significantly enhanced the preference for social novelty (Figure 4). Importantly,over the anxiolytic, antidepressant, anti-manic and social behavior-enhancing oral dose ranges tested, l-THP did not induce any significant alteration in the level of locomotor activity, or any significant myorelaxant effect based on the horizontal wire test (Figure 6). These results suggest that l-THP represents a candidate therapeutic agent for the treatment of anxiety, depression and bipolar disorders.
Although dl-THP also elicited anxiolytic and antidepressant effects on mice, its minimum effective dosage was ten-fold that of l-THP (1.0 mg/kg versus 0.1 mg/kg) for anxiolysis (Figure 1), and four-fold that of l-THP (2.0 mg/kg versus 0.5 mg/kg) for reduction of immobility time (Figure 3). The lack of any significant effect by d-THP in either case would explain in part the greater efficacy of l-THP compared to dl-THP. In addition, pharmacokinetic and pharmacodynamic differences between l-THP and dl-THP could also be a contributing factor. In rats, it was reported that the concentration of l-THP in plasma and different brain regions was 3-5 fold that of dl-THP [19], and the jejunum absorption rate of l-THP was about 20% higher than that of dl-THP [20].
As well, since there were close to equal amounts of d-THP and l-THP in dl-THP (Supplementary Figure8), and d-THP was devoid of any anxiolytic or antidepressant activity in the tested dose ranges, it could not be ruled out that d-THP might antagonize to some extent the anxiolytic and antidepression actions of l-THP. Notably, it has been found that l-THP and d-THP exert different effects in the central nervous system, with d-THP acting as a dopamine depletor in the striatum, and l-THP as a non-selective dopamine receptor antagonist [21]. While l-THP interacts with the GABAA, alpha-1 adrenergic, alpha-2 adrenergic and 5-HT1A receptors [22], the receptor-activity profile of d-THP or dl-THP has not been investigated as widely. Thus, different profiles of l-THP and dl-THP in their interactions with various neuroreceptors may also be a factor for their dissimilar anxiolytic and antidepressant effects.
Previously, it was found that flumazenil antagonized fully or partially the anxiolytic effect induced by dl-THP in mice [8], or rats [9]. Figure 2 shows the extensive inhibition of both the anxiolytic and antidepressant effects of l-THP and the antidepressant effect of dl-THP by co-administration of 1.25 mg/kg i.p.flumazenil, suggesting that these effects could be mediated by the BZ site of GABAA receptors. However, l-THP inhibited the binding of [3H]-spiperone, a ligand of dopamine D2-like receptors, to rat cerebral cortex membranes with Ki of 5.10μM, but inhibited [3H]-flunitrazepam, a ligand of the BZ site of GABAA receptors, with a much higher Ki of 368 μM (Figure 7). Therefore, although the inhibition of anxiolytic and antidepressant actions of l-THP by flumazenil pointed to the mediation of these actions by GABAA receptors, the more efficient binding of l-THP to dopamine D2-like receptors compared to GABAA receptors suggests that l-THP might bind to dopamine D2-like receptors that activate downstream GABAA receptors to induce the anxiolytic and antidepressant actions, possibly with some participation of GABAA-mediated astrocytic gliotransmission in neuronal functions [23,24].
Conclusion
The present study demonstrated that l-THP exerted anxiolytic, antidepressant and anti-manic like effects on mice. Antidepressants in current usage include selective serotonin reuptake inhibitors/serotonin-norepinephrine reuptake inhibitors (SSRI/SNRI), which require 2-4 weeks to produce therapeutic effects. In view of this, patients may require treatment with anxiolytic benzodiazepines to achieve rapid symptom relief before the SSRI/SNRI becomes effective [25-27]. Since l-THP is fast-acting on anxiety assessed by the elevated plus-maze test (Figure 1), depression assessed by the tail-suspension test (Figure3) or Crawley’s three-chamber behavioral tests (Figure 4), as well as manic-like behavior (Figure 5) unaccompanied by significant side effects in the form of altered locomotor activity or muscle weakness (Figure 6), it merits further investigation as a fast-acting candidate agent for the treatment of anxiety, depression or bipolar disorder.
Acknowledgement
This study was supported by grants from the Innovation and Technology Commission of the Government of the Hong Kong Special Administrative Region (No. ITCPD/17-9), and the Ministry of Science and Technology of China (the National Science and Technology Major Project No. 2017ZX09301064). The authors are grateful to Ms. Peggy Lee for expert assistance.
Conflict of Interest
A Provisional US Patent Application based on findings described in this paper has been filed by PharmacoGenetics Limited of the Hong Kong University of Science and Technology Entrepreneurship Program./p>
References
- Chang CK, Lin MT. 2001. DL-Tetrahydropalmatine may act through inhibition of amygdaloid release of dopamine to inhibit an epileptic attack in rats. Neurosci. Lett.307: 163-166.[Ref.]
- Lin MT, Chueh FY, Hsieh MT, et al. 1996. Antihypertensive effects of dl-tetrahydropalmatine: an active principle isolated from Corydalis. Clin. Exp. Pharmacol. Physiol. 23: 738-742.[Ref.]
- Kin KC, Zhen XF, Hsu B. 1964. Studies on the pharmacological actions of corydalis XII. the effects of optical isomers of tetrahydropalmatine (THP) on central nervous system. Acta. Physiologica Sinica. 27: 47-58.[Ref.]
- Liu YL, Liang JH, Yan LD, et al. 2005. Effects of l-tetrahydropalmatine on locomotor sensitization to oxycodone in mice. Acta. Pharmacol. Sin. 26: 533-538.[Ref.]
- Mantsch JR, Li SJ, Risinger R, et al.2007. Levo-tetrahydropalmatine attenuates cocaine self-administration and cocaine-induced reinstatement in rats. Psychopharmacology. 192: 581-591.[Ref.]
- Zhao N, Chen Y, Zhu J, et al. 2014. Levo-tetrahydropalmatine attenuates the development and expression of methamphetamine-induced locomotor sensitization and the accompanying activation of ERK in the nucleus accumbens and caudate putamen in mice. Neuroscience. 258: 101-110.[Ref.]
- Lee B, Sur B, Yeom M, et al. 2014. L-tetrahydropalmatine ameliorates development of anxiety and depression-related symptoms induced by single prolonged stress in rats. Biomol. Ther (Seoul). 22: 213-222.[Ref.]
- Leung WC, Zheng H, Huen M, et al. 2003. Anxiolytic-like action of orally administered dl-tetrahydropalmatine in elevated plus-maze. Prog. Neuropsychopharmacol Biol. Psychiatry. 27: 775-779.[Ref.]
- Henkes, H, Franz M, Kendall O,et al. 2011. Evaluation of the anxiolytic properties of tetrahydropalmatine, a Corydalis yanhusuo compound, in the male Sprague-Dawley rat. AANA. Journal. 79: 75-80.[Ref.]
- Wong LW, Kan JW, Nguyen TH, et al. 2015. Bis(mandelato)borate: an effective, inexpensive spiroborate anion for chiral resolution. Chem. Commun. (Camb.). 51: 15760-15763. [Ref.]
- Ren L, Wang F, Xu Z, et al. 2010. GABA(A) receptor subtype selectivity underlying anxiolytic effect of 6-hydroxyflavone. Biochem. Pharmacol. 79: 1337-1344.[Ref.]
- Kalueff AV, Tuohimaa P. 2004. Experimental modeling of anxiety and depression. Acta. neurobiologiae experimentalis. 64: 439-448.[Ref.]
- Treit D, Menard J, Royan C. 1993. Anxiogenic stimuli in the elevated plus-maze. Pharmacol. Biochem. Behav. 44: 463-469.[Ref.]
- Kaidanovich-Beilin O, Lipina T, Vukobradovic I, et al. 2011. Assessment of social interaction behaviors.J. Vis. Exp. 48: 2473.[Ref.]
- Pathak G, Ibrahim BA, McCarthy SA, et al. 2015. Amphetamine sensitization in mice is sufficient to produce both manic- and depressive-related behaviors as well as changes in the functional connectivity of corticolimbic structures. Neuropharmacology. 95:434-447.[Ref.]
- Vogel HW. 2002. Benzodiazepine receptor: [3H]-flunitrazepam binding assay. In: Vogel HG, Vogel HW (eds).Drug Discovery and Evaluation: Pharmacology Assay. Springer: New York, pp. 408-409.[Ref.]
- Huen MS, Hui KM, Leung JW, et al. 2003. Naturally occurring 2'-hydroxyl-substituted flavonoids as high-affinity benzodiazepine site ligands. Biochem. Pharmacol. 66: 2397-2407.[Ref.]
- Chatterjee TK, Scott CE, Vazquez DM, et al. 1988. Interaction of [3H] spiperone with rat striatal dopamine D-2 receptors: kinetic evidence for antagonist-induced formation of ternary complex. Mol. Pharmacol. 33: 402-413.[Ref.]
- Hong Z, Fan G, Le J, Chai Y, et al. 2006. Brain pharmacokinetics and tissue distribution of tetrahydropalmatine enantiomers in rats after oral administration of the racemate. Biopharm Drug Dispos. 27: 111-117.[Ref.]
- Wu PS, Huang SD, Ye YJ, et al. 2007. Difference absorption of l-tetrahydropalmatine and dl-tetrahydropalmatine in intestine of rats. Yao Xue Xue Bao.42:, 534-537.[Ref.]
- Jin GZ, Xu SX, Yu LP. 1986. Effects of tetrahydropalmatine enantiomers on dopamine receptor subtypes in brain. Sci. Sin(B). 29: 1054-1057.[Ref.]
- Wang JB, Mantsch JR. 2012. L-tetrahydropalamatine: a potential new medication for the treatment of cocaine addiction. Future Med. Chem. 4: 177-186.[Ref.]
- Bovolin P, Santi MR, Puia G, et al. 1992. Expression patterns of gamma-aminobutyric acid type A receptor subunit mRNAs in primary cultures of granule neurons and astrocytes from neonatal rat cerebella. Proc Natl Acad Sci USA. 89: 9344-9348.[Ref.]
- Halassa MM, Fellin T, Haydon P.G. 2006. The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol Med. 13: 54-63.[Ref.]
- Stein DJ. 2001. Comorbidity in generalized anxiety disorder: impact and implications. J. Clin. Psychiatry. 62(Suppl 8). 29-36.[Ref.]
- Stein MB. 2003. Attending to anxiety disorders in primary care. J. Clin. Psychiatry. 64(Suppl 15). 35-39.[Ref.]
- Dunlop BW, Davis PG. 2008. Combination treatment with benzodiazepines and SSRIs for comorbid anxiety and depression: a review. Prim. Care Companion J Clin Psychiatry. 10: 222-228.[Ref.]




















